Details of the Active Pharmaceutical Ingredient (API)
| General Information of API (ID: D00125) | |||||
|---|---|---|---|---|---|
| Name |
Chlorpropamide
|
||||
| Synonyms |
Click to Show/Hide the Synonyms of This API
chlorpropamide; 94-20-2; Chloropropamide; Diabinese; Chlorpropamid; Diabenese; Glucamide; Meldian; Chlorodiabina; Chloronase; Diabeneza; Diabetoral; Adiaben; Catanil; Diabaril; Dynalase; Insulase; Melitase; Diabechlor; Diabenal; Mellinese; Millinese; Asucrol; Glisema; Oradian; Diabet-Pages; Diamel Ex; 4-chloro-N-(propylcarbamoyl)benzenesulfonamide; Clorpropamide; Diabexan; Prodiaben; 1-(4-Chlorophenylsulfonyl)-3-propylurea; Chlorpropamidum; Clorpropamida; 1-(p-Chlorobenzenesulfonyl)-3-propylurea; 1-Propyl-3-(p-chlorobenzenesulfonyl)urea; N-(4-Chlorophenylsulfonyl)-N'-propylurea; N-(p-Chlorobenzenesulfonyl)-N'-propylurea; N-Propyl-N'-(p-chlorobenzenesulfonyl)urea; 4-chloro-N-[(propylamino)carbonyl]benzenesulfonamide; 1-(4-chlorophenyl)sulfonyl-3-propylurea; 1-(p-Chlorophenylsulfonyl)-3-propylurea; 1-p-Chlorophenyl-3-(propylsulfonyl)urea; NCI-C01752; 4-Chloro-N-((propylamino)carbonyl)benzenesulfonamide; UNII-WTM2C3IL2X; N-Propyl-N'-p-chlorphenylsulfonylcarbamide; n-Propyl-N'-p-chlorophenylsulfonylcarbamide; NSC 44634; Benzenesulfonamide, 4-chloro-N-[(propylamino)carbonyl]-; U-9818; 4-Chloro-4-((propylamino)carbonyl)benzenesulfonamide; Benzenesulfonamide, 4-chloro-N-((propylamino)carbonyl)-; Clorpropamid; MFCD00079004; WTM2C3IL2X; CHEMBL498; MLS000028395; CHEBI:3650; Bioglumin; Insogen; Urea, 1-((p-chlorophenyl)sulfonyl)-3-propyl-; NSC626720; CAS-94-20-2; NCGC00015216-11; Chlorporpamide; SMR000058364; Clorpropamide [DCIT]; DSSTox_CID_322; chlorpropamide, alpha-form; Clorpropamide [Italian]; 1-(4-chloro-benzenesulfonyl)-3-n-propyl-urea; Urea, 1-[(p-chlorophenyl)sulfonyl]-3-propyl-; chlorpropamide, epsilon-form; DSSTox_RID_75512; DSSTox_GSID_20322; chlorpropamide, epsilon`-form; Chlorpropamidum [INN-Latin]; Clorpropamida [INN-Spanish]; Diabinese (TN); CCRIS 155; 1-(p-Chlorobenzensulfonyl)-3-propylurea; HSDB 2051; 1-((p-Chlorophenyl)sulfonyl)-3-propylurea; 4-chloro-n-(propylaminocarbonyl)benzenesulfonamide; SR-01000000060; 4-chloro-N-((propylaminocarbonyl)benzenesulfonamide; N-[(4-chlorophenyl)sulfonyl](propylamino)carboxamide; EINECS 202-314-5; U-3818; NSC 626720; BRN 2218363; 1-[(4-chlorobenzene)sulfonyl]-3-propylurea; Urea, 1-((p-chloropenyl)sulfonyl)-3-propyl-; Prestwick_684; Chlorpropamide [USP:INN:BAN:JAN]; Chlorpropamide B.P.; Spectrum_000144; ACMC-209rqj; 1-(4-chlorobenzenesulfonyl)-3-propylurea; 1-(4-chlorophenyl)sulfonyl-3-propyl-urea; Opera_ID_359; ADENYLOSUCCINICACID; Prestwick0_000323; Prestwick1_000323; Prestwick2_000323; Prestwick3_000323; Spectrum2_000089; Spectrum3_000347; Spectrum4_000284; Spectrum5_000719; WLN: GR DSWMVM3; Lopac-C-1290; chlorpropamide, delta-form; C 1290; Lopac0_000229; SCHEMBL23947; BSPBio_000325; BSPBio_002013; KBioGR_000808; KBioGR_002273; KBioSS_000624; KBioSS_002274; MLS001148665; DivK1c_000513; SPECTRUM1500185; SPBio_000018; SPBio_002246; BPBio1_000359; GTPL6801; DTXSID9020322; HMS501J15; KBio1_000513; KBio2_000624; KBio2_002273; KBio2_003192; KBio2_004841; KBio2_005760; KBio2_007409; KBio3_001233; KBio3_002753; Chlorpropamide (JP17/USP/INN); cMAP_000007; NINDS_000513; HMS1569A07; HMS1920M05; HMS2091E08; HMS2096A07; HMS2233L19; HMS3259A17; HMS3260N19; HMS3373D09; HMS3428C03; HMS3652L03; HMS3713A07; Pharmakon1600-01500185; BCP09162; HY-B1429; NSC44634; ZINC1530599; Tox21_110102; Tox21_201391; Tox21_302789; Tox21_500229; ANW-40217; BDBM50344965; CCG-38905; NSC-44634; NSC756690; SBB056923; STK857458; AKOS001482739; Tox21_110102_1; CS-4917; DB00672; KS-5316; LP00229; MCULE-3261763364; NC00503; NSC-626720; NSC-756690; SDCCGSBI-0050217.P005; IDI1_000513; MRF-0000539; NCGC00015216-01; NCGC00015216-02; NCGC00015216-03; NCGC00015216-04; NCGC00015216-05; NCGC00015216-06; NCGC00015216-07; NCGC00015216-08; NCGC00015216-09; NCGC00015216-10; NCGC00015216-12; NCGC00015216-13; NCGC00015216-14; NCGC00015216-17; NCGC00015216-18; NCGC00015216-23; NCGC00021451-03; NCGC00021451-04; NCGC00021451-05; NCGC00021451-06; NCGC00021451-07; NCGC00021451-08; NCGC00256414-01; NCGC00258942-01; NCGC00260914-01; AK116029; SY052508; SBI-0050217.P004; AB00051944; Chlorpropamide, analytical standard, >=97%; EU-0100229; ST50412138; SW196839-3; A16447; D00271; Urea, 1-propyl-3-(p-chloro-benzenesulfonyl)-; AB00051944_16; AB00051944_17; Q1075324; SR-01000000060-2; SR-01000000060-4; SR-01000000060-6; W-100205; 4-Chloro-N-[(propylamino)-carbonyl]benzenesulfonamide; BRD-K97746869-001-05-6; BRD-K97746869-001-15-5; 1-Chloro-4-(([(propylamino)carbonyl]amino)sulfonyl)benzene #; Chlorpropamide, European Pharmacopoeia (EP) Reference Standard; Chlorpropamide, United States Pharmacopeia (USP) Reference Standard; Chlorpropamide, Pharmaceutical Secondary Standard; Certified Reference Material
|
||||
| Clinical Status |
Approved
|
||||
| Disease Indication | Diabetes mellitus | ICD-11: 5A10 | [1] | ||
| PubChem CID | |||||
| Formula |
C10H13ClN2O3S
|
||||
| Canonical SMILES |
CCCNC(=O)NS(=O)(=O)C1=CC=C(C=C1)Cl
|
||||
| InChI |
1S/C10H13ClN2O3S/c1-2-7-12-10(14)13-17(15,16)9-5-3-8(11)4-6-9/h3-6H,2,7H2,1H3,(H2,12,13,14)
|
||||
| InChIKey |
RKWGIWYCVPQPMF-UHFFFAOYSA-N
|
||||
| Click to Show/Hide the Molecular Data (Structure/Property) of This API | |||||
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=2727"></iframe>
|
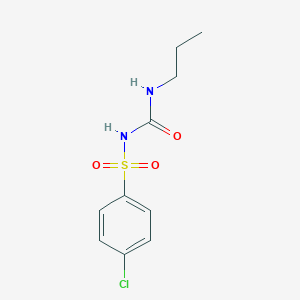 |
|||
| 3D MOL | 2D MOL | ||||
| Physicochemical Properties | Molecular Weight | 276.74 | Topological Polar Surface Area | 83.6 | |
| XlogP | 2.3 | Complexity | 345 | ||
| Heavy Atom Count | 17 | Rotatable Bond Count | 4 | ||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 3 | ||
| Full List of Drug Formulations (DFMs) Containing This API | ||||||
|---|---|---|---|---|---|---|
| Chlorpropamide 100 mg tablet | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Glycine; Sodium lauryl sulfate; Calcium carbonate; D&c yellow no. 10; Fd&c blue no. 1; Magnesium stearate; Croscarmellose sodium; Silicon dioxide; Cellulose, microcrystalline
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | Mylan Pharamceuticals | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| FD&C blue no. 1 | DIG Info | Solute carrier SLCO2B1 (Ki = 13 uM) | [2] | |||
| Sodium lauryl sulfate | DIG Info | Solute carrier SLCO2B1 (Ki = 1.98 uM) | [2] | |||
| Calcium carbonate | DIG Info | Carbonic anhydrase IX (Ki = 8600 nM) | [3] | |||
| Quinoline yellow WS | DIG Info | Estrogen receptor alpha (IC50 = 18 uM) | [4] | |||
| Aminoethanoic acid | DIG Info | Glycine type-1 transporter (IC50 = 31600 nM) | [5] | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [6] | |||
| Carmellose sodium | DIG Info | Albendazole monooxygenase (Protein expression upregulation) | [6] | |||
| Silicon dioxide | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [6] | |||
| Chlorpropamide 250 mg tablet | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Glycine; Sodium lauryl sulfate; Calcium carbonate; D&c yellow no. 10; Fd&c blue no. 1; Magnesium stearate; Croscarmellose sodium; Silicon dioxide; Cellulose, microcrystalline
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | Mylan Pharamceuticals; PD-Rx Pharmaceuticals | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| FD&C blue no. 1 | DIG Info | Solute carrier SLCO2B1 (Ki = 13 uM) | [2] | |||
| Sodium lauryl sulfate | DIG Info | Solute carrier SLCO2B1 (Ki = 1.98 uM) | [2] | |||
| Calcium carbonate | DIG Info | Carbonic anhydrase IX (Ki = 8600 nM) | [3] | |||
| Quinoline yellow WS | DIG Info | Estrogen receptor alpha (IC50 = 18 uM) | [4] | |||
| Aminoethanoic acid | DIG Info | Glycine type-1 transporter (IC50 = 31600 nM) | [5] | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [6] | |||
| Carmellose sodium | DIG Info | Albendazole monooxygenase (Protein expression upregulation) | [6] | |||
| Silicon dioxide | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [6] | |||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.
