Details of the Active Pharmaceutical Ingredient (API)
| General Information of API (ID: D00254) | |||||
|---|---|---|---|---|---|
| Name |
Ethosuximide
|
||||
| Synonyms |
Click to Show/Hide the Synonyms of This API
ethosuximide; 77-67-8; Zarontin; Etosuximida; 2-Ethyl-2-methylsuccinimide; Ethosuxide; Ethosuccimide; Ethosuccinimide; Etosuximid; Petnidan; Suxinutin; Atysmal; Emeside; Ethymal; Suxilep; Pentinimid; Peptinimid; Petinimid; Pyknolepsinum; Succimitin; Zaraondan; Capitus; Mesentol; Pemalin; Simatin; Succimal; Suximal; Thetamid; Zarodan; Zarondan; Zartalin; Asamid; Etomal; Ronton; Aethosuximide; Piknolepsin; Thilopemal; Epileo Petit MAL; 3-Ethyl-3-methylpyrrolidine-2,5-dione; Zarondan-Saft; Simatin(E); Ethosuximidum; 3-Ethyl-3-methyl-2,5-pyrrolidinedione; 3-Methyl-3-ethylsuccinimide; 2-Methyl-2-ethylsuccinimide; 2,5-Pyrrolidinedione, 3-ethyl-3-methyl-; CI-366; Succinimide, 2-ethyl-2-methyl-; 3-Ethyl-3-methylsuccinimide; PM 671; PM-671; CN-10,395; 3-Methyl-3-ethylpyrrolidine-2,5-dione; alpha-Ethyl-alpha-methylsuccinimide; alpha-Methyl-alpha-ethylsuccinimide; gamma-Methyl-gamma-ethyl-succinimide; C.I. 366; Cl 366; (+-)-2-Ethyl-2-methylsuccinimide; CN-10395; gamma-ethyl-gamma-methyl-succinimide; NSC-64013; 3-Ethyl-3-methylpyrroline-2,5-dione; CHEMBL696; .alpha.-Ethyl-.alpha.-methylsuccinimide; 3-ethyl-3-methyl-pyrrolidine-2,5-dione; CHEBI:4887; Aethosuccimidum; Etosuximide; NSC64013; MFCD00072123; .gamma.-Methyl-.gamma.-ethylsuccinimide; Etosuccimide; Etosuccimide [DCIT]; Aethosuximide [German]; DSSTox_CID_3019; N-Ethyl methylsuccinimide; DSSTox_RID_76832; DSSTox_GSID_23019; Ethosuximidum [INN-Latin]; Etosuximida [INN-Spanish]; Pyknole.psi.num; Piknole.psi.n; Zarontin (TN); HSDB 1119; SR-01000075863; EINECS 201-048-7; CI 366; NSC 64013; BRN 0117054; CAS-77-67-8; NCGC00016320-01; Prestwick_611; Zorontin,Ethosuximide; Ethosuximide [USAN:USP:INN:BAN:JAN]; Spectrum_001385; Prestwick0_000165; Prestwick1_000165; Prestwick2_000165; Prestwick3_000165; Spectrum2_001483; Spectrum3_000944; Spectrum4_001051; Spectrum5_001073; (.+/-.)-Ethosuximide; 2, 3-ethyl-3-methyl-; E 7138; NCIOpen2_000014; Lopac0_000532; SCHEMBL34212; BSPBio_000029; KBioGR_001342; KBioSS_001865; 5-21-09-00595 (Beilstein Handbook Reference); DivK1c_000218; SPECTRUM1502196; 2,5-Pyrrolidinedione, 3-ethyl-3-methyl-, (+-)-; SPBio_001465; SPBio_001950; BPBio1_000033; GTPL7182; WLN: T5VMVTJ D2 D1; DTXSID7023019; SCHEMBL20541518; Ethosuximide (JP17/USP/INN); Ethosuximide, analytical standard; HMS500K20; KBio1_000218; KBio2_001865; KBio2_004433; KBio2_007001; KBio3_002008; AOB5337; NINDS_000218; HMS1568B11; HMS1921L14; HMS2092D20; HMS2095B11; HMS3261L05; HMS3712B11; HMS3885D12; Pharmakon1600-01502196; HY-B1378; Ethosuximide 1.0 mg/ml in Methanol; Tox21_110370; Tox21_500532; (+/-)-2-ethyl-2-methylsuccinimide; ANW-41860; BDBM50240424; CCG-39217; NSC758192; 3-ethyl-3-methylazolidine-2,5-dione; AKOS005261742; Tox21_110370_1; CS-7976; DB00593; LP00532; MCULE-4034034545; NE54559; NSC-758192; SDCCGSBI-0050515.P004; .alpha.-Methyl-.alpha.-ethylsuccinimide; IDI1_000218; NCGC00015418-02; NCGC00015418-03; NCGC00015418-04; NCGC00015418-05; NCGC00015418-06; NCGC00015418-08; NCGC00015418-09; NCGC00015418-15; NCGC00093923-01; NCGC00093923-02; NCGC00093923-03; NCGC00093923-04; NCGC00261217-01; 3-Ethyl-3-methyl-2, 5-pyrrolidinedione; AS-16859; SBI-0050515.P003; 2, 5-Pyrrolidinedione, 3-ethyl-3-methyl-; AB00052288; AK01050738; EU-0100532; FT-0668060; FT-0668061; ST51037248; C07505; D00539; AB00052288_04; zarontin3-Ethyl-3-methyl-pyrrolidine-2,5-dione; Q421567; SR-01000075863-1; SR-01000075863-3; SR-01000075863-5; W-109273; 3-Ethyl-3-methyl-pyrrolidine-2,5-dione(Ethosuximide); BRD-A99633051-001-04-7; BRD-A99633051-001-05-4; 3-Ethyl-3-methyl-pyrrolidine-2,5-dione (ethosuximide); Z2379802739; 3-ethyl-5-hydroxy-3-methyl-3,4-dihydro-2H-pyrrol-2-one; 4-ethyl-5-hydroxy-4-methyl-3,4-dihydro-2H-pyrrol-2-one; Ethosuximide, European Pharmacopoeia (EP) Reference Standard; Ethosuximide, United States Pharmacopeia (USP) Reference Standard
|
||||
| Clinical Status |
Approved
|
||||
| Disease Indication | Epilepsy | ICD-11: 8A60 | [1] | ||
| PubChem CID | |||||
| Formula |
C7H11NO2
|
||||
| Canonical SMILES |
CCC1(CC(=O)NC1=O)C
|
||||
| InChI |
1S/C7H11NO2/c1-3-7(2)4-5(9)8-6(7)10/h3-4H2,1-2H3,(H,8,9,10)
|
||||
| InChIKey |
HAPOVYFOVVWLRS-UHFFFAOYSA-N
|
||||
| Click to Show/Hide the Molecular Data (Structure/Property) of This API | |||||
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=3291"></iframe>
|
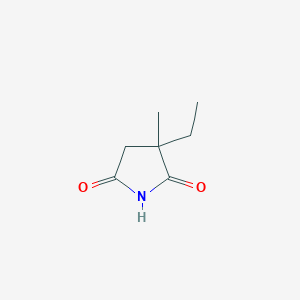 |
|||
| 3D MOL | 2D MOL | ||||
| Physicochemical Properties | Molecular Weight | 141.17 | Topological Polar Surface Area | 46.2 | |
| XlogP | 0.4 | Complexity | 188 | ||
| Heavy Atom Count | 10 | Rotatable Bond Count | 1 | ||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 2 | ||
| Full List of Drug Formulations (DFMs) Containing This API | ||||||
|---|---|---|---|---|---|---|
| Ethosuximide 250 mg capsule | Click to Show/Hide the Full List of Formulation(s): 5 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Methylparaben; Fd&c red no. 40; Butyl alcohol; Fd&c yellow no. 6; Isopropyl alcohol; Propylparaben; Sorbitol; Ammonia; Glycerin; Propylene glycol; Titanium dioxide; Silicon dioxide; Dimethicone; Gelatin; Mineral oil; Polyethylene glycols; Shellac
|
|||||
| Dosage Form | Oral Capsule | |||||
| Company | Heritage Pharmaceuticals; Zydus Pharmaceuticals | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Allura red AC dye | DIG Info | Solute carrier SLCO2B1 (Ki = 4.7 uM) | [2] | |||
| methylparaben | DIG Info | Carbonic anhydrase VII (Ki = 780 nM) | [3] | |||
| Sunset yellow FCF | DIG Info | Solute carrier SLCO2B1 (Ki = 68.4 uM) | [4] | |||
| Allura red AC dye | DIG Info | Solute carrier SLCO2B1 (Ki = 2.59 uM) | [4] | |||
| Propyl 4-hydroxybenzoate | DIG Info | Estrogen receptor alpha (IC50 = 11 uM) | [2] | |||
| Isopropyl alcohol | DIG Info | Lymphoma P388/ADR cells (IC50 = 0.22 uM) | [5] | |||
| Propylene glycol | DIG Info | Albendazole monooxygenase (Inhibition ratio < 20 %) | [6] | |||
| Silicon dioxide | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [7] | |||
| Drug Formulation 2 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Fd&c yellow no. 6; Sorbitol; Glycerin; Fd&c red no. 3; Polyethylene glycol 400; Gelatin
|
|||||
| Dosage Form | Oral Capsule | |||||
| Company | Avera McKennan Hospital; VersaPharm | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Sunset yellow FCF | DIG Info | Solute carrier SLCO2B1 (Ki = 68.4 uM) | [4] | |||
| FD&C red no. 3 | DIG Info | Phosphodiesterase 3A (IC50 = 0.092 uM) | [2] | |||
| Polyethylene glycol 400 | DIG Info | Albendazole monooxygenase (IC50 = 10.77 mg.mL-1) | [6] | |||
| Drug Formulation 3 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
D&c yellow no. 10; Sorbitol; Glycerin; Fd&c red no. 3; Polyethylene glycol 400; Gelatin
|
|||||
| Dosage Form | Oral Capsule | |||||
| Company | Greenstone; Parke-Davis Div of Pfizer Inc | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Quinoline yellow WS | DIG Info | Estrogen receptor alpha (IC50 = 18 uM) | [2] | |||
| FD&C red no. 3 | DIG Info | Phosphodiesterase 3A (IC50 = 0.092 uM) | [2] | |||
| Polyethylene glycol 400 | DIG Info | Albendazole monooxygenase (IC50 = 10.77 mg.mL-1) | [6] | |||
| Drug Formulation 4 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Fd&c yellow no. 6; Glycerin; Water; Gelatin; Polyethylene glycols
|
|||||
| Dosage Form | Oral Capsule | |||||
| Company | Banner Life Sciences; Bionpharma | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Sunset yellow FCF | DIG Info | Solute carrier SLCO2B1 (Ki = 68.4 uM) | [4] | |||
| Drug Formulation 5 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Fd&c yellow no. 6; Glycerin; Gelatin; Polyethylene glycols
|
|||||
| Dosage Form | Oral Capsule | |||||
| Company | Pliva | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Sunset yellow FCF | DIG Info | Solute carrier SLCO2B1 (Ki = 68.4 uM) | [4] | |||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.
