Details of the Active Pharmaceutical Ingredient (API)
| General Information of API (ID: D00485) | |||||
|---|---|---|---|---|---|
| Name |
Olaparib
|
||||
| Synonyms |
Click to Show/Hide the Synonyms of This API
Olaparib; 763113-22-0; AZD2281; Lynparza; KU-0059436; AZD 2281; AZD-2281; Olaparib (AZD2281, Ku-0059436); UNII-WOH1JD9AR8; KU-59436; AZD2281(olaparib); MFCD13185161; WOH1JD9AR8; Olaparib (AZD-2281); C24H23FN4O3; CHEBI:83766; AZ2281; 937799-91-2; KU59436; OLAPARIB cpd; Olaparib [USAN:INN]; Acylpiperazine analogue, 47; Olaparibum; Lynparza (TN); PubChem19148; KU 59436; Olaparib (AZD2281); Olaparib - AZD2281; AZD-2281 (Olaparib); Olaparib (JAN/USAN/INN); MLS006010185; SCHEMBL426568; CHEMBL521686; GTPL7519; BDBM27566; AOB1085; DTXSID60917988; EX-A002; BCPP000360; HMS3295I09; HMS3426C03; HMS3654G13; HMS3746K07; HMS3870H03; BCP01872; ABP000423; ANW-43329; NSC747856; NSC753686; ZINC40430143; AKOS005145764; AC-7939; ACN-032229; AZ-2281; BCP9000363; CCG-264799; CS-0075; DB09074; EX-7210; KEYLYNK-010 COMPONENT OLAPARIB; NSC-747856; NSC-753686; QC-2660; SB14617; SS-4573; AZD2281,Olaparib, KU-0059436; NCGC00238451-01; NCGC00238451-02; NCGC00238451-09; NCGC00238451-11; AK-40514; BR-40514; HY-10162; Olaparib (AZD2281, KU0059436); SMR004701291; SY040527; OLAPARIB COMPONENT OF KEYLYNK-010; AB0009952; Olaparib(AZD2281,KuDOSKU-0059436); BB 0260909; FT-0651458; KU 0059436; SW218142-2; EC-000.2324; D09730; S-3781; S-7836; Z-3275; AZD2281(olaparib)/AZD-2281/KU0059436; J-503540; Q7083106; BRD-K02113016-001-08-9; BRD-K02113016-001-09-7; AZD 2281; KU 0059436; KU 59436; 1-(cyclopropylcarbonyl)-4-[5-[(3,4-dihydro-4-oxo-1-phthalazine; 1021843-02-6
|
||||
| Clinical Status |
Approved
|
||||
| Disease Indication | Ovarian cancer | ICD-11: 2C73 | [1] | ||
| PubChem CID | |||||
| Formula |
C24H23FN4O3
|
||||
| Canonical SMILES |
C1CC1C(=O)N2CCN(CC2)C(=O)C3=C(C=CC(=C3)CC4=NNC(=O)C5=CC=CC=C54)F
|
||||
| InChI |
1S/C24H23FN4O3/c25-20-8-5-15(14-21-17-3-1-2-4-18(17)22(30)27-26-21)13-19(20)24(32)29-11-9-28(10-12-29)23(31)16-6-7-16/h1-5,8,13,16H,6-7,9-12,14H2,(H,27,30)
|
||||
| InChIKey |
FDLYAMZZIXQODN-UHFFFAOYSA-N
|
||||
| Click to Show/Hide the Molecular Data (Structure/Property) of This API | |||||
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=23725625"></iframe>
|
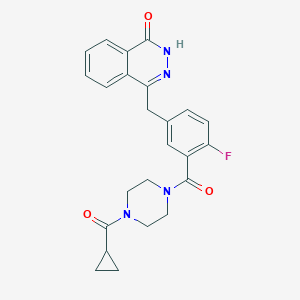 |
|||
| 3D MOL | 2D MOL | ||||
| Physicochemical Properties | Molecular Weight | 434.5 | Topological Polar Surface Area | 82.1 | |
| XlogP | 1.9 | Complexity | 790 | ||
| Heavy Atom Count | 32 | Rotatable Bond Count | 4 | ||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 5 | ||
| Full List of Drug Formulations (DFMs) Containing This API | ||||||
|---|---|---|---|---|---|---|
| Olaparib 100 mg tablet | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Sodium stearyl fumarate; Mannitol; Ferric oxide yellow; Ferrosoferric oxide; Titanium dioxide; Water; Polyethylene glycol 400; Silicon dioxide; Copovidone k25-31; Hypromelloses
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | AstraZeneca | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Mannitol | DIG Info | Glycine receptor alpha-1 (EC50 = 12589.25 nM) | [2] | |||
| Sodium stearyl fumarate | DIG Info | Leukemia K562 cells (IC50 = 20.2 ug.mL-1) | [3] | |||
| Polyethylene glycol 400 | DIG Info | Albendazole monooxygenase (IC50 = 10.77 mg.mL-1) | [4] | |||
| Silicon dioxide | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [5] | |||
| Olaparib 150 mg tablet | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Sodium stearyl fumarate; Mannitol; Ferric oxide yellow; Ferrosoferric oxide; Titanium dioxide; Water; Polyethylene glycol 400; Silicon dioxide; Copovidone k25-31; Hypromelloses
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | AstraZeneca | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Mannitol | DIG Info | Glycine receptor alpha-1 (EC50 = 12589.25 nM) | [2] | |||
| Sodium stearyl fumarate | DIG Info | Leukemia K562 cells (IC50 = 20.2 ug.mL-1) | [3] | |||
| Polyethylene glycol 400 | DIG Info | Albendazole monooxygenase (IC50 = 10.77 mg.mL-1) | [4] | |||
| Silicon dioxide | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [5] | |||
| Olaparib 50 mg capsule | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Potassium acetate; Butyl alcohol; Isopropyl alcohol; Ammonia; Ferrosoferric oxide; Propylene glycol; Titanium dioxide; Gellan gum (low acyl); Hypromelloses; Lauroyl peg-32 glycerides; Shellac
|
|||||
| Dosage Form | Oral Capsule | |||||
| Company | AstraZeneca | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Potassium acetate | DIG Info | Free fatty acid receptor 3 (EC50 = 5000 nM) | [6] | |||
| Isopropyl alcohol | DIG Info | Lymphoma P388/ADR cells (IC50 = 0.22 uM) | [7] | |||
| Propylene glycol | DIG Info | Albendazole monooxygenase (Inhibition ratio < 20 %) | [4] | |||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.
