| Synonyms |
Click to Show/Hide the Synonyms of This API
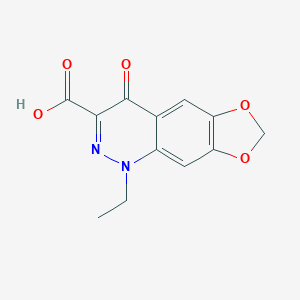
cinoxacin; 28657-80-9; Cinobac; Compound 64716; Cinobactin; Cinoxacine; Cinoxacino; Cinoxacinum; Uronorm; Cinx; UNII-LMK22VUH23; 1-Ethyl-6,7-methylenedioxy-4(1H)-oxocinnoline-3-carboxylic acid; NSC 304467; MLS000069630; 1-ethyl-4-oxo-[1,3]dioxolo[4,5-g]cinnoline-3-carboxylic acid; LMK22VUH23; 1-Ethyl-1,4-dihydro-4-oxo(1,3)dioxolo(4,5-g)cinnoline-3-carboxylic acid; 1-Ethyl-1,4-dihydro-4-oxo[1,3]dioxolo[4,5-g]cinnoline-3-carboxylic acid; SMR000058232; [1,3]Dioxolo[4,5-g]cinnoline-3-carboxylic acid, 1-ethyl-1,4-dihydro-4-oxo-; CHEBI:3716; 1-Ethyl-4-oxo-1,4-dihydro[1,3]dioxolo[4,5-g]cinnoline-3-carboxylic acid; NSC304467; NSC-304467; NCGC00015277-02; CAS-28657-80-9; DSSTox_CID_2822; (1,3)Dioxolo(4,5-g)cinnoline-3-carboxylic acid, 1,4-dihydro-1-ethyl-4-oxo-; (1,3)Dioxolo(4,5-g)cinnoline-3-carboxylic acid, 1-ethyl-1,4-dihydro-4-oxo-; 1-Ethyl-4-oxo-1,4-dihydro-[1,3]dioxolo[4,5-g]cinnoline-3-carboxylic acid; DSSTox_RID_76745; Cinoxacine [INN-French]; Cinoxacinum [INN-Latin]; DSSTox_GSID_22822; Cinoxacino [INN-Spanish]; Lilly 64716; 1-ethyl-4-oxo-1H,4H,7H-[1,3]dioxolo[4,5-g]cinnoline-3-carboxylic acid; 1-ethyl-4-oxohydro-7H-1,3-dioxoleno[4,5-g]cinnoline-3-carboxylic acid; Cinobac (TN); SR-01000000129; EINECS 249-133-8; BRN 1084304; Noxigram; CCRIS 8206; 1-ethyl-1,4-dihydro-4-oxo-[1,3]dioxolo[4,5-g]cinnoline-3-carboxylic acid; TNP00246; Prestwick_239; Cinoxacin [USAN:USP:INN:BAN:JAN]; PubChem16235; Spectrum_000152; Opera_ID_1392; Prestwick0_000780; Prestwick1_000780; Prestwick2_000780; Prestwick3_000780; Spectrum2_000570; Spectrum3_000352; Spectrum4_000289; Spectrum5_000749; Lopac-C-8645; C 8645; cid_2762; CHEMBL1208; Lopac0_000309; Oprea1_131085; SCHEMBL43770; BSPBio_000860; BSPBio_002043; KBioGR_000818; KBioSS_000632; MLS001148076; DivK1c_000318; SPECTRUM1500190; SPBio_000360; SPBio_002799; Cinoxacin (JP17/USP/INN); BPBio1_000946; DTXSID8022822; BDBM39350; HMS500P20; KBio1_000318; KBio2_000632; KBio2_003200; KBio2_005768; KBio3_001263; ZINC32350; NINDS_000318; HMS1570K22; HMS1920O11; HMS2091E18; HMS2097K22; HMS2235K07; HMS3260N20; HMS3370B19; HMS3714K22; Pharmakon1600-01500190; HY-B1085; Tox21_110121; Tox21_500309; 2323AH; CCG-39160; MFCD00056776; NSC756695; SBB003082; AKOS022507372; Tox21_110121_1; CS-4652; DB00827; LP00309; MCULE-3390574434; NSC-756695; SDCCGSBI-0050297.P005; IDI1_000318; NCGC00015277-01; NCGC00015277-03; NCGC00015277-04; NCGC00015277-05; NCGC00015277-06; NCGC00015277-07; NCGC00015277-08; NCGC00015277-09; NCGC00015277-10; NCGC00015277-11; NCGC00015277-12; NCGC00015277-13; NCGC00015277-14; NCGC00015277-17; NCGC00015277-21; NCGC00023754-03; NCGC00023754-04; NCGC00023754-05; NCGC00023754-06; NCGC00023754-07; NCGC00260994-01; AS-73151; ST044511; SBI-0050297.P004; DB-047427; EU-0100309; FT-0602947; SW196546-3; C08052; D00872; AB00051948_17; AB00051948_18; A819523; J-017182; Q1639588; SR-01000000129-2; SR-01000000129-4; SR-01000000129-7; BRD-K14704277-001-05-1; BRD-K14704277-001-15-0; 1-Ethyl-1,3]dioxolo[4,5-g]cinnoline-3-carboxylic acid; Cinoxacin, United States Pharmacopeia (USP) Reference Standard; [1,5-g]cinnoline-3-carboxylic acid, 1-ethyl-1,4-dihydro-4-oxo-; 1-Ethyl-1,4-dihydro-4-oxo(1,3)dioxolo(4,5-g)cinnoline-3-carboxylicacid; 1-ethyl-4-oxidanylidene-[1,3]dioxolo[4,5-g]cinnoline-3-carboxylic acid; 5-Ethyl-8-oxo-5,8-dihydro-1,3-dioxa-5,6-diaza-cyclopenta[b]naphthalene-7-carboxylic acid
|
 click to show the detail info of this DFM
click to show the detail info of this DFM