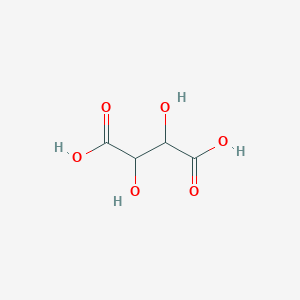
| Synonyms |
Click to Show/Hide the Synonyms of This DIG
DL-Tartaric acid; tartaric acid; 2,3-Dihydroxysuccinic acid; 2,3-Dihydroxybutanedioic acid; 133-37-9; 526-83-0; Racemic acid; Uvic acid; Traubensaure; Racemic tartaric acid; DL-Tartrate; Paratartaric acid; Paratartaric aicd; Threaric acid; Resolvable tartaric acid; BUTANEDIOIC ACID, 2,3-DIHYDROXY-; (+)-Tartaric acid; Natural tartaric acid; Acidum tartaricum; NSC62778; Tartaric acid D,L; Tartaric acid, L-(+)-; Baros; CHEBI:15674; dl-2,3-dihydroxybutanedioic acid; Dextrotartaric acid; (2RS,3RS)-Tartaric acid; tartrate; MFCD00071626; NSC 148314; Butanedioic acid, 2,3-dihydroxy-, (2R,3R)-rel-; E-7050 (2S,3S)-2,3-dihydroxysuccinic acid; (2R,3R)-rel-2,3-Dihydroxysuccinic acid; DL-Tartaric acid, 99.5%; Butanedioic acid, 2,3-dihydroxy-(R*,R*)-(.+/-.)-; Butanedioic acid, 2,3-dihydroxy-, (R*,R*)-; 868-14-4; Tartaric acid, L-; (2R,3R)-2,3-Dihydroxybernsteinsaeure; Tartaric acid (VAN); 1007601-97-9; Kyselina vinna [Czech]; NSC155080; Butanedioic acid, 2,3-dihydroxy- (2R,3R)-; Tartaric acid [USAN:JAN]; (.+-.)-Tartaric acid; C4H6O6; (+)-(2R,3R)-Tartaric acid; d-alpha,beta-Dihydroxysuccinic acid; NSC-62778; Kyselina 2,3-dihydroxybutandiova [Czech]; (+) tartaric acid; (-) tartaric acid; 1,2-Dihydroxyethane-1,2-dicarboxylic acid; AI3-06298; 1,2-dicarboxylic acid; WLN: QVYQYQVQ; (-) D-Tartaric acid; ACMC-209qpg; Sal tartar (Salt/Mix); Tartaric acid, (DL)-; Butanedioic acid, 2,3-dihydroxy- (R-(R*,R*))-; Butanedioic acid, 2,3-dihydroxy-, [S-(R*,R*)]-; Malic acid, 3-hydroxy-; laevo-(+)-tartaric acid; dextro,laevo-tartaric acid; Succinic acid,3-dihydroxy; SCHEMBL848; ACMC-209cz3; bmse000167; Succinic acid,3-dihydroxy-; (.+/-.)-Tartaric acid; DSSTox_CID_26986; DSSTox_RID_82036; 2,3-dihydroxy-succinic acid; DSSTox_GSID_46986; Oprea1_827092; TARTARIC ACID, (L); Tartaric acid, (.+-.)-; Butanedioic acid,3-dihydroxy-; CHEMBL333714; Dihydroxysuccinic acid, (DL)-; Tartaric acid, (.+/-.)-; DTXSID5046986; L+Tartaric Acid FCC, NF, USP; 2,3-bis(oxidanyl)butanedioic acid; HMS3370M15; DL-TARTARIC-2,3-D2 ACID; (+)-2,3-dihydroxybutanedioic acid; (S,S)-Tartaric acid;Tartaric acid; BCP14303; Tox21_302052; BBL011588; MFCD00064206; NSC133735; NSC148314; NSC608773; s2997; STK387106; 2,3-Dihydroxysuccinic acid, (DL)-; 3-carboxy-2,3-dihydroxypropanoic acid; AKOS000120086; AKOS016844048; MCULE-3867000095; NE11122; NSC-133735; NSC-148314; NSC-608773; SMP2_000051; d-.alpha.,.beta.-Dihydroxysuccinic acid; NCGC00256063-01; NCGC00347131-03; AK105884; AK116146; AS-10983; CAS-133-37-9; NCI60_001102; (+)-2,3-dihydroxy-1,4-butanedioic acid; DB-016129; DB-016159; DB-042899; AM20110247; FT-0624346; FT-0625514; FT-0628018; FT-0628243; FT-0656080; FT-0772946; FT-0773804; (+/-)-2,3-dihydroxy-1,4-butanedioic acid; 1467-EP2269610A2; 1467-EP2269986A1; 1467-EP2269988A2; 1467-EP2269989A1; 1467-EP2269990A1; 1467-EP2270003A1; 1467-EP2270006A1; 1467-EP2270008A1; 1467-EP2270011A1; 1467-EP2270014A1; 1467-EP2270505A1; 1467-EP2272516A2; 1467-EP2272537A2; 1467-EP2272822A1; 1467-EP2272827A1; 1467-EP2272835A1; 1467-EP2272843A1; 1467-EP2272844A1; 1467-EP2275401A1; 1467-EP2275411A2; 1467-EP2275413A1; 1467-EP2275414A1; 1467-EP2277507A1; 1467-EP2277848A1; 1467-EP2277858A1; 1467-EP2277866A1; 1467-EP2277867A2; 1467-EP2280003A2; 1467-EP2280009A1; 1467-EP2281559A1; 1467-EP2281563A1; 1467-EP2281817A1; 1467-EP2281819A1; 1467-EP2281823A2; 1467-EP2284149A1; 1467-EP2284160A1; 1467-EP2284169A1; 1467-EP2284178A2; 1467-EP2284179A2; 1467-EP2286795A1; 1467-EP2287147A2; 1467-EP2287154A1; 1467-EP2287155A1; 1467-EP2287156A1; 1467-EP2287160A1; 1467-EP2287161A1; 1467-EP2287162A1; 1467-EP2289510A1; 1467-EP2289518A1; 1467-EP2289879A1; 1467-EP2289883A1; 1467-EP2289885A1; 1467-EP2289890A1; 1467-EP2289893A1; 1467-EP2292227A2; 1467-EP2292231A1; 1467-EP2292234A1; 1467-EP2292592A1; 1467-EP2292611A1; 1467-EP2292612A2; 1467-EP2292617A1; 1467-EP2292619A1; 1467-EP2295055A2; 1467-EP2295402A2; 1467-EP2295406A1; 1467-EP2295414A1; 1467-EP2295416A2; 1467-EP2295418A1; 1467-EP2295424A1; 1467-EP2295433A2; 1467-EP2298731A1; 1467-EP2298734A2; 1467-EP2298735A1; 1467-EP2298742A1; 1467-EP2298746A1; 1467-EP2298747A1; 1467-EP2298748A2; 1467-EP2298755A1; 1467-EP2298758A1; 1467-EP2298759A1; 1467-EP2298763A1; 1467-EP2298767A1; 1467-EP2298768A1; 1467-EP2298772A1; 1467-EP2298777A2; 1467-EP2298779A1; 1467-EP2301544A1; 1467-EP2301922A1; 1467-EP2301931A1; 1467-EP2301937A1; 1467-EP2301940A1; 1467-EP2305219A1; 1467-EP2305248A1; 1467-EP2305257A1; 1467-EP2305633A1; 1467-EP2305636A1; 1467-EP2305641A1; 1467-EP2305646A1; 1467-EP2305651A1; 1467-EP2305653A1; 1467-EP2305655A2; 1467-EP2305659A1; 1467-EP2305663A1; 1467-EP2305664A1; 1467-EP2305672A1; 1467-EP2305673A1; 1467-EP2305675A1; 1467-EP2305676A1; 1467-EP2305679A1; 1467-EP2305683A1; 1467-EP2308839A1; 1467-EP2308841A2; 1467-EP2308849A1; 1467-EP2308850A1; 1467-EP2308851A1; 1467-EP2308854A1; 1467-EP2308857A1; 1467-EP2308861A1; 1467-EP2308869A1; 1467-EP2308871A1; 1467-EP2308872A1; 1467-EP2308873A1; 1467-EP2308875A1; 1467-EP2311453A1; 1467-EP2311801A1; 1467-EP2311802A1; 1467-EP2311803A1; 1467-EP2311807A1; 1467-EP2311809A1; 1467-EP2311810A1; 1467-EP2311811A1; 1467-EP2311818A1; 1467-EP2311821A1; 1467-EP2311831A1; 1467-EP2311834A1; 1467-EP2311837A1; 1467-EP2311839A1; 1467-EP2311842A2; 1467-EP2314295A1; 1467-EP2314574A1; 1467-EP2314575A1; 1467-EP2314576A1; 1467-EP2314584A1; 1467-EP2314585A1; 1467-EP2314586A1; 1467-EP2314587A1; 1467-EP2314588A1; 1467-EP2314589A1; 1467-EP2314593A1; 1467-EP2316457A1; 1467-EP2316458A1; 1467-EP2316459A1; 1467-EP2316470A2; 1467-EP2316825A1; 1467-EP2316826A1; 1467-EP2316827A1; 1467-EP2316828A1; 1467-EP2316829A1; 1467-EP2316831A1; 1467-EP2316832A1; 1467-EP2316833A1; 1467-EP2316834A1; 1467-EP2316835A1; 1467-EP2316836A1; 1467-EP2316837A1; 1467-EP2371814A1; 1467-EP2374454A1; 1467-EP2374780A1; 1467-EP2374781A1; 1467-EP2380874A2; A22830; A22866; Butanedioic acid,3-dihydroxy- [R-(R*,R*)]-; 133D379; A829202; Q194322; Butanedioic acid,3-dihydroxy-, (R*,R*)-(.+-.)-; F2191-0230; Z1258943354; 1,2-Dihydroxyethane-1,2-dicarboxylic acid;2,3-Dihydrosuccinic acid; (2S,3S)-(-)-Tartaric acid; D(-)-Threaric acid;D(-)-Dihydroxysuccinic acid; Copper, mixt. with [R-(R*,R*)]-2,3-dihydroxybutanedioic acid monopotassium salt; 91469-46-4
|