Details of the Active Pharmaceutical Ingredient (API)
| General Information of API (ID: D00258) | |||||
|---|---|---|---|---|---|
| Name |
Etoposide
|
||||
| Synonyms |
Click to Show/Hide the Synonyms of This API
etoposide; VePesid; 33419-42-0; Toposar; trans-Etoposide; Lastet; (-)-Etoposide; Zuyeyidal; Etoposido; Etoposidum; Etoposidum [INN-Latin]; Etoposide (VP16); VP 16-213; Vepesid J; VP-16-213; 4-Demethylepipodophyllotoxin beta-D-ethylideneglucoside; UNII-6PLQ3CP4P3; NSC-141540; VP 16 (pharmaceutical); 4'-Demethylepipodophyllotoxin 9-(4,6-O-(R)-ethylidene-beta-D-glucopyranoside); 6PLQ3CP4P3; Epipodophyllotoxin VP-16213; CHEMBL44657; CHEBI:4911; NK 171; Demethylepipodophyllotoxin-beta-D-ethylideneglucoside; NSC 141540; 4'-Demethylepipodophyllotoxin 9-(4,6-O-ethylidene-beta-D-glucopyranoside); Etosid; [1,3]benzodioxol-8-one; Etoposido [INN-Spanish]; Etopophos (phosphate salt); Vepeside; Etopol; VP 16213; MFCD00869325; NSC141540; Etoposide (VP-16); SMR000112002; CCRIS 2392; HSDB 6517; VePESID (TN); EINECS 251-509-1; 4'-Demethylepipodophyllotoxin ethylidene-.beta.-D-glucoside; Etoposide,(S); 4'-O-Demethyl-1-O-(4,6-O-ethylidene-beta-D-glucopyranosyl)epipodophyllotoxin; Epipodophyllotoxin, 4'-demethyl-, 9-(4,6-O-ethylidene-beta-D-glucopyranoside); Etoposide [USAN:USP:INN:BAN:JAN]; Etoposide; VP-16; CPD000112002; Epipodophyllotoxin-beta-D-ethyliden-glucoside, 4'-demethyl-; Prestwick3_000396; SCHEMBL4259; BSPBio_000611; MLS000049957; MLS001074951; MLS001424283; MLS002153463; MLS002207239; MLS002222184; Etoposide (JP17/USP/INN); BPBio1_000673; GTPL6815; DTXSID5023035; etoposide4-o-b-d-galactopyranoside; HMS2052N05; HMS2089F14; HMS2096O13; HMS2232L03; HMS3713O13; EX-A1207; ZINC3938684; BDBM50127140; Etoposide - CAS 33419-42-0; AKOS007930275; AB07572; ACN-057122; BCP9000669; CCG-101165; CS-1774; DB00773; EBD2157958; Etoposide, synthetic, >=98%, powder; NC00415; SDCCGSBI-0050405.P002; 4'-Demethyl-epipodophyllotoxin 9-[4,6-O-(R)-ethylidene-beta-D-glucopyranoside; Epipodophyllotoxin, 4'-demethyl-, 4,6-O-ethylidene-beta-D-glucopyranoside (8CI); NCGC00179504-02; AS-35312; HY-13629; SBI-0051910.P002; AB00438905; A-8109; C01576; D00125; 13165-EP2269989A1; 13165-EP2270008A1; 13165-EP2270014A1; 13165-EP2270018A1; 13165-EP2272827A1; 13165-EP2272832A1; 13165-EP2275413A1; 13165-EP2275420A1; 13165-EP2277565A2; 13165-EP2277566A2; 13165-EP2277567A1; 13165-EP2277568A2; 13165-EP2277569A2; 13165-EP2277570A2; 13165-EP2277865A1; 13165-EP2277876A1; 13165-EP2280012A2; 13165-EP2281815A1; 13165-EP2287156A1; 13165-EP2289892A1; 13165-EP2292280A1; 13165-EP2292614A1; 13165-EP2292615A1; 13165-EP2292617A1; 13165-EP2295055A2; 13165-EP2295416A2; 13165-EP2295426A1; 13165-EP2295427A1; 13165-EP2298305A1; 13165-EP2298746A1; 13165-EP2298748A2; 13165-EP2298764A1; 13165-EP2298765A1; 13165-EP2298768A1; 13165-EP2298772A1; 13165-EP2298778A1; 13165-EP2298780A1; 13165-EP2301928A1; 13165-EP2301933A1; 13165-EP2305640A2; 13165-EP2305642A2; 13165-EP2305671A1; 13165-EP2305679A1; 13165-EP2305689A1; 13165-EP2308812A2; 13165-EP2308833A2; 13165-EP2308839A1; 13165-EP2308855A1; 13165-EP2308861A1; 13165-EP2311453A1; 13165-EP2311807A1; 13165-EP2311808A1; 13165-EP2311825A1; 13165-EP2311827A1; 13165-EP2311829A1; 13165-EP2311840A1; 13165-EP2311842A2; 13165-EP2314574A1; 13165-EP2316832A1; 13165-EP2316833A1; 13165-EP2316834A1; 13165-EP2374454A1; AB00438905-17; AB00438905-18; AB00438905_19; 419E420; Q418817; SR-01000763196; SR-01000763196-3; BRD-K37798499-001-02-5; BRD-K37798499-001-05-8; BRD-K37798499-001-10-8; BRD-K37798499-001-14-0; BRD-K37798499-001-27-2; Etoposide, British Pharmacopoeia (BP) Reference Standard; Etoposide, European Pharmacopoeia (EP) Reference Standard; Etoposide, United States Pharmacopeia (USP) Reference Standard; 4''-Demethylepipodophyllotoxin 9-(4,6-O-(R)-ethylidene-beta-D-glucopyranoside); Etoposide for system suitability, European Pharmacopoeia (EP) Reference Standard; 121471-01-0
|
||||
| Clinical Status |
Approved
|
||||
| Disease Indication | Testicular carcinoma | ICD-11: 2C80 | [1] | ||
| PubChem CID | |||||
| Formula |
C29H32O13
|
||||
| Canonical SMILES |
C[C@@H]1OC[C@@H]2[C@@H](O1)[C@@H]([C@H]([C@@H](O2)O[C@H]3[C@H]4COC(=O)[C@@H]4[C@@H](C5=CC6=C(C=C35)OCO6)C7=CC(=C(C(=C7)OC)O)OC)O)O
|
||||
| InChI |
1S/C29H32O13/c1-11-36-9-20-27(40-11)24(31)25(32)29(41-20)42-26-14-7-17-16(38-10-39-17)6-13(14)21(22-15(26)8-37-28(22)33)12-4-18(34-2)23(30)19(5-12)35-3/h4-7,11,15,20-22,24-27,29-32H,8-10H2,1-3H3/t11-,15+,20-,21-,22+,24-,25-,26-,27-,29+/m1/s1
|
||||
| InChIKey |
VJJPUSNTGOMMGY-MRVIYFEKSA-N
|
||||
| Click to Show/Hide the Molecular Data (Structure/Property) of This API | |||||
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=36462"></iframe>
|
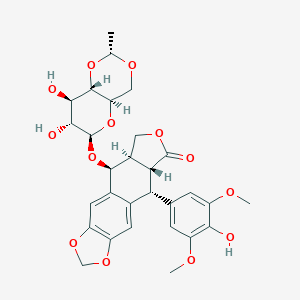 |
|||
| 3D MOL | 2D MOL | ||||
| Physicochemical Properties | Molecular Weight | 588.6 | Topological Polar Surface Area | 161 | |
| XlogP | 0.6 | Complexity | 969 | ||
| Heavy Atom Count | 42 | Rotatable Bond Count | 5 | ||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 13 | ||
| Full List of Drug Formulations (DFMs) Containing This API | ||||||
|---|---|---|---|---|---|---|
| Etoposide 50 mg capsule | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Anhydrous citric acid; Fd&c red no. 40; Fd&c blue no. 1; Ferric oxide red; Glycerin; Propylene glycol; Titanium dioxide; Gelatin; Hypromelloses; Polyethylene glycols
|
|||||
| Dosage Form | Oral Capsule | |||||
| Company | Mylan Pharamceuticals | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Allura red AC dye | DIG Info | Solute carrier SLCO2B1 (Ki = 4.7 uM) | [2] | |||
| FD&C blue no. 1 | DIG Info | Solute carrier SLCO2B1 (Ki = 13 uM) | [3] | |||
| Kyselina citronova | DIG Info | Perilipin-1 (IC50 = 3708 nM) | [4] | |||
| Allura red AC dye | DIG Info | Solute carrier SLCO2B1 (Ki = 2.59 uM) | [3] | |||
| Propylene glycol | DIG Info | Albendazole monooxygenase (Inhibition ratio < 20 %) | [5] | |||
| Etoposide Phosphate eq 100mg base/vial injectable | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Sodium Citrate; Dextran 40
|
|||||
| Dosage Form | Injectable | |||||
| Company | E.R. Squibb & Sons L.L.C.; H2-Pharma | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Sodium citrate anhydrous | DIG Info | Carbonic anhydrase IV (Ki = 99 nM) | [6] | |||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.
