Details of the Active Pharmaceutical Ingredient (API)
| General Information of API (ID: D00510) | |||||
|---|---|---|---|---|---|
| Name |
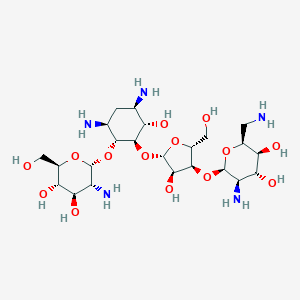
Paromomycin
|
||||
| Synonyms |
Click to Show/Hide the Synonyms of This API
paromomycin; Aminosidin; AMINOSIDINE; Hydroxymycin; Zygomycin A1; Crestomycin; Paucimycin; Estomycin; Monomycin A; 7542-37-2; catenulin; Paromomycin I; Neomycin E; Paramomycin Sulfate; Humatin; Paromomicina; Paromomycinum; Paucimycinum; Paromomycine; UNII-61JJC8N5ZK; Gabbromycin; Paromomycin (INN); Paromomycin [INN]; (1R,2R,3S,4R,6S)-4,6-diamino-2-{[3-O-(2,6-diamino-2,6-dideoxy-beta-L-idopyranosyl)-beta-D-ribofuranosyl]oxy}-3-hydroxycyclohexyl 2-amino-2-deoxy-alpha-D-glucopyranoside; Antibiotic 503-3; 61JJC8N5ZK; Amminosidin; Antibiotic SF 767B; Gabromycin; Humycin; Aminosidine I; Quintomycin C; CHEBI:7934; Antibiotic 2230D; paramomycin sulphate; Hydroxymycin sulfate; Paramomycin; Monomycin; Paromomycin (TN); Paromomycin sulfate Rx346208; (2S,3S,4R,5R,6R)-5-amino-2-(aminomethyl)-6-(((2R,3S,4R,5S)-5-(((1R,2R,3S,5R,6S)-3,5-diamino-2-(((2S,3R,4R,5S,6R)-3-amino-4,5-dihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)-6-hydroxycyclohexyl)oxy)-4-hydroxy-2-(hydroxymethyl)tetrahydrofuran-3-yl)oxy)tetrahydro-2H-pyran-3,4-diol; Aminosidine, sulfate; hATT & Paromomycin; Paromomycin [INN:BAN]; Paromomycine [INN-French]; Paromomycinum [INN-Latin]; Paromomicina [INN-Spanish]; (2S,3S,4R,5R,6R)-5-amino-2-(aminomethyl)-6-[(2R,3S,4R,5S)-5-[(1R,2R,3S,5R,6S)-3,5-diamino-2-[(2S,3R,4R,5S,6R)-3-amino-4,5-dihydroxy-6-(hydroxymethyl)tetrahydropyran-2-yl]oxy-6-hydroxy-cyclohexoxy]-4-hydroxy-2-(hydroxymethyl)tetrahydrofuran-3-yl]oxy-tetrahydropyran-3,4-diol; EINECS 231-423-0; BRN 0072285; Human .alpha.-1-antitrypsin & Paromomyin; SCHEMBL4072; 4-18-00-07534 (Beilstein Handbook Reference); CHEMBL370143; DTXSID8023424; ZINC60183170; AKOS030489917; DB01421; MCULE-1528618434; NCGC00166210-02; D-Streptamine, O-2-amino-2-deoxy-alpha-D-glucopyranosyl-(1-4)-O-(O-2,6-diamino-2,6-dideoxy-beta-L-idopyranosyl-(1-3)-beta-D-ribofuranosyl-(1-5))-2-deoxy-; O-2-Amino-2-deoxy-.alpha.-D-glucopyranosyl-(1->4)-O-[O-2,6-diamino-2,6-dideoxy-.beta.-L-idopyranosyl-(1->3)-.beta.D-ribofuranosyl(1->5)]-2-deoxy-D-streptamine; C00832; D07467; 10845-EP2301536A1; 10845-EP2301538A1; 10845-EP2311455A1; 10845-EP2316452A1; AB00639998_04; Q415625; PAROMOMYCIN I; AMMINOSIDIN; CATENULIN; CRESTOMYCIN; MONOMYCIN A; NEOMYCIN E; (2R,3S,4R,5R,6S)-5-amino-6-{[(1R,2R,3S,4R,6S)-4,6-diamino-2-{[(2S,3R,4S,5R)-4-{[(2R,3R,4R,5S,6S)-3-amino-6-(aminomethyl)-4,5-dihydroxyoxan-2-yl]oxy}-3-hydroxy-5-(hydroxymethyl)oxolan-2-yl]oxy}-3-hydroxycyclohexyl]oxy}-2-(hydroxymethyl)oxane-3,4-diol; Streptamine, O-2,6-diamino-2,6-dideoxy-beta-L-idopyranosyl-(1-3)-O-beta-D-ribofuranosyl-(1-5)-O-(2-amino-2-deoxy-alpha-D-glucopyranosyl-(1-4))-2-deoxy-
|
||||
| Clinical Status |
Approved
|
||||
| Disease Indication | Amebiasis | ICD-11: 1A36 | [1] | ||
| PubChem CID | |||||
| Formula |
C23H45N5O14
|
||||
| Canonical SMILES |
C1[C@H]([C@@H]([C@H]([C@@H]([C@H]1N)O[C@@H]2[C@@H]([C@H]([C@@H]([C@H](O2)CO)O)O)N)O[C@H]3[C@@H]([C@@H]([C@H](O3)CO)O[C@@H]4[C@@H]([C@H]([C@@H]([C@@H](O4)CN)O)O)N)O)O)N
|
||||
| InChI |
1S/C23H45N5O14/c24-2-7-13(32)15(34)10(27)21(37-7)41-19-9(4-30)39-23(17(19)36)42-20-12(31)5(25)1-6(26)18(20)40-22-11(28)16(35)14(33)8(3-29)38-22/h5-23,29-36H,1-4,24-28H2/t5-,6+,7+,8-,9-,10-,11-,12+,13-,14-,15-,16-,17-,18-,19-,20-,21-,22-,23+/m1/s1
|
||||
| InChIKey |
UOZODPSAJZTQNH-LSWIJEOBSA-N
|
||||
| Click to Show/Hide the Molecular Data (Structure/Property) of This API | |||||
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=165580"></iframe>
|
 |
|||
| 3D MOL | 2D MOL | ||||
| Physicochemical Properties | Molecular Weight | 615.6 | Topological Polar Surface Area | 347 | |
| XlogP | -8.7 | Complexity | 870 | ||
| Heavy Atom Count | 42 | Rotatable Bond Count | 9 | ||
| Hydrogen Bond Donor Count | 13 | Hydrogen Bond Acceptor Count | 19 | ||
| Full List of Drug Formulations (DFMs) Containing This API | ||||||
|---|---|---|---|---|---|---|
| Paromomycin 250 mg capsule | Click to Show/Hide the Full List of Formulation(s): 3 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
D&c red no. 28; D&c yellow no. 10; Fd&c red no. 40; Fd&c blue no. 1; Fd&c blue no. 2; Ferrosoferric oxide; Propylene glycol; Titanium dioxide; Gelatin; Shellac
|
|||||
| Dosage Form | Oral Capsule | |||||
| Company | Department of State Health Services, Pharmacy Branch; Heritage Pharmaceuticals | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Allura red AC dye | DIG Info | Solute carrier SLCO2B1 (Ki = 4.7 uM) | [2] | |||
| FD&C blue no. 1 | DIG Info | Solute carrier SLCO2B1 (Ki = 13 uM) | [3] | |||
| FD&C blue no. 2 | DIG Info | Adenosine receptor A3 (IC50 = 1 uM) | [2] | |||
| D&C red no. 28 | DIG Info | Organic anion transporter 1 (Ki = 0.064 uM) | [2] | |||
| Quinoline yellow WS | DIG Info | Estrogen receptor alpha (IC50 = 18 uM) | [2] | |||
| Allura red AC dye | DIG Info | Solute carrier SLCO2B1 (Ki = 2.59 uM) | [3] | |||
| Propylene glycol | DIG Info | Albendazole monooxygenase (Inhibition ratio < 20 %) | [4] | |||
| Drug Formulation 2 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
D&c red no. 28; D&c yellow no. 10; Fd&c red no. 40; Fd&c blue no. 1; Fd&c blue no. 2; Ferrosoferric oxide; Propylene glycol; Titanium dioxide; Gelatin, unspecified; Shellac
|
|||||
| Dosage Form | Oral Capsule | |||||
| Company | Central Texas Community Health Centers | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Allura red AC dye | DIG Info | Solute carrier SLCO2B1 (Ki = 4.7 uM) | [2] | |||
| FD&C blue no. 1 | DIG Info | Solute carrier SLCO2B1 (Ki = 13 uM) | [3] | |||
| FD&C blue no. 2 | DIG Info | Adenosine receptor A3 (IC50 = 1 uM) | [2] | |||
| D&C red no. 28 | DIG Info | Organic anion transporter 1 (Ki = 0.064 uM) | [2] | |||
| Quinoline yellow WS | DIG Info | Estrogen receptor alpha (IC50 = 18 uM) | [2] | |||
| Allura red AC dye | DIG Info | Solute carrier SLCO2B1 (Ki = 2.59 uM) | [3] | |||
| Propylene glycol | DIG Info | Albendazole monooxygenase (Inhibition ratio < 20 %) | [4] | |||
| Drug Formulation 3 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Fd&c yellow no. 5; Titanium dioxide; Fd&c green no. 3; Gelatin
|
|||||
| Dosage Form | Oral Capsule | |||||
| Company | Sun Pharmaceutical Industries | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Hydrazine yellow | DIG Info | GABA(A) receptor alpha-1 (IC50 = 13 uM) | [2] | |||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.
