Details of the Active Pharmaceutical Ingredient (API)
| General Information of API (ID: D00526) | |||||
|---|---|---|---|---|---|
| Name |
Phenoxybenzamine
|
||||
| Synonyms |
Click to Show/Hide the Synonyms of This API
phenoxybenzamine; Dibenyline; Dibenzyline; Dibenylin; 59-96-1; Bensylyt; Benzylyt; Fenoxibenzamina; Fenossibenzamina [DCIT]; Fenossibenzamina; Fenoxibenzamina [INN-Spanish]; Phenoxybenzaminum [INN-Latin]; Benzenemethanamine, N-(2-chloroethyl)-N-(1-methyl-2-phenoxyethyl)-; N-benzyl-N-(2-chloroethyl)-1-phenoxypropan-2-amine; NSC 37448; 2-(N-Benzyl-2-chloroethylamino)-1-phenoxypropane; N-(2-Chloroethyl)-N-(1-methyl-2-phenoxyethyl)benzenemethanamine; Benzyl(2-chloroethyl)-(1-methyl-2-phenoxyethyl)amine; N-(2-Chloroethyl)-N-(1-methyl-2-phenoxyethyl)benzylamine; Dibenylene; CHEMBL753; CHEBI:8077; 59-96-1 (free); Benzylamine, N-(2-chloroethyl)-N-(1-methyl-2-phenoxyethyl)-; NCGC00015121-08; Bensylyte; Phenoxybenzaminum; DSSTox_CID_3458; DSSTox_RID_77034; benzyl(2-chloroethyl)(1-phenoxypropan-2-yl)amine; DSSTox_GSID_23458; Phenoxybenzamine [INN:BAN]; CAS-59-96-1; CCRIS 505; HSDB 4005; Phenoxybenzamine (INN); EINECS 200-446-8; N-Phenoxyisopropyl-N-benzyl-beta-chloroethylamine; BRN 2129697; Spectrum_000378; Prestwick0_000944; Prestwick1_000944; Prestwick2_000944; Prestwick3_000944; Spectrum2_001815; Spectrum4_000769; Spectrum5_001370; SCHEMBL5722; Lopac0_000235; BSPBio_000908; BSPBio_001278; BSPBio_002356; KBioGR_000618; KBioGR_001158; KBioSS_000618; KBioSS_000858; 4-12-00-02204 (Beilstein Handbook Reference); DivK1c_000800; SPBio_001829; SPBio_003067; BPBio1_001000; GTPL7268; DTXSID0023458; KBio1_000800; KBio2_000618; KBio2_000858; KBio2_003186; KBio2_003426; KBio2_005754; KBio2_005994; KBio3_001095; KBio3_001096; NINDS_000800; Bio2_000479; Bio2_000959; HMS1362P19; HMS1792P19; HMS1990P19; HMS2089J09; HMS3403P19; N-(2-chloroethyl)-N-(phenylmethyl)-1-(phenyloxy)propan-2-amine; Tox21_110087; BDBM50017679; AKOS015961144; Tox21_110087_1; CCG-204330; DB00925; MCULE-1084779069; SDCCGSBI-0050223.P004; IDI1_000800; IDI1_002234; MRF-0000619; NCGC00015121-03; NCGC00015121-04; NCGC00015121-05; NCGC00015121-06; NCGC00015121-07; NCGC00015121-09; NCGC00015121-11; NCGC00015121-12; NCGC00015121-13; NCGC00015121-14; NCGC00015121-16; NCGC00015121-17; NCGC00089748-03; NCGC00089748-04; NCGC00089748-05; NCGC00089748-07; AC-13214; SBI-0050223.P003; AB00053702; FT-0778642; C07435; D08358; AB00053702-12; AB00053702_13; AB00053702_14; L001197; Q419824; N-Phenoxyisopropyl-N-benzyl-.beta.-chloroethylamine; Q-201556; N-benzyl-N-(2-chloroethyl)-1-phenoxy-propan-2-amine; N-Benzyl-N-(2-chloroethyl)-1-phenoxy-2-propanamine #; Benzyl-(2-chloro-ethyl)-(1-methyl-2-phenoxy-ethyl)-amine; 102737-84-8
|
||||
| Clinical Status |
Approved
|
||||
| Disease Indication | Essential hypertension | ICD-11: BA00 | [1] | ||
| PubChem CID | |||||
| Formula |
C18H22ClNO
|
||||
| Canonical SMILES |
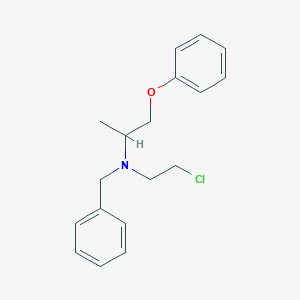
CC(COC1=CC=CC=C1)N(CCCl)CC2=CC=CC=C2
|
||||
| InChI |
1S/C18H22ClNO/c1-16(15-21-18-10-6-3-7-11-18)20(13-12-19)14-17-8-4-2-5-9-17/h2-11,16H,12-15H2,1H3
|
||||
| InChIKey |
QZVCTJOXCFMACW-UHFFFAOYSA-N
|
||||
| Click to Show/Hide the Molecular Data (Structure/Property) of This API | |||||
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=4768"></iframe>
|
 |
|||
| 3D MOL | 2D MOL | ||||
| Physicochemical Properties | Molecular Weight | 303.8 | Topological Polar Surface Area | 12.5 | |
| XlogP | 4.4 | Complexity | 262 | ||
| Heavy Atom Count | 21 | Rotatable Bond Count | 8 | ||
| Hydrogen Bond Donor Count | 0 | Hydrogen Bond Acceptor Count | 2 | ||
| Full List of Drug Formulations (DFMs) Containing This API | ||||||
|---|---|---|---|---|---|---|
| Phenoxybenzamine 10 mg capsule | Click to Show/Hide the Full List of Formulation(s): 4 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Anhydrous lactose; Sodium lauryl sulfate; Fd&c red no. 40; Butyl alcohol; Isopropyl alcohol; Ammonia; Ferrosoferric oxide; Propylene glycol; Titanium dioxide; Water; Silicon dioxide; Gelatin; Shellac
|
|||||
| Dosage Form | Oral Capsule | |||||
| Company | West-Ward Pharmaceticals | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Allura red AC dye | DIG Info | Solute carrier SLCO2B1 (Ki = 4.7 uM) | [2] | |||
| Sodium lauryl sulfate | DIG Info | Solute carrier SLCO2B1 (Ki = 1.98 uM) | [3] | |||
| Allura red AC dye | DIG Info | Solute carrier SLCO2B1 (Ki = 2.59 uM) | [3] | |||
| Isopropyl alcohol | DIG Info | Lymphoma P388/ADR cells (IC50 = 0.22 uM) | [4] | |||
| Propylene glycol | DIG Info | Albendazole monooxygenase (Inhibition ratio < 20 %) | [5] | |||
| Silicon dioxide | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [6] | |||
| Drug Formulation 2 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Lactose; Sodium lauryl sulfate; D&c red no. 33; Fd&c yellow no. 6; Fd&c red no. 3; Silicon dioxide; Gelatin
|
|||||
| Dosage Form | Oral Capsule | |||||
| Company | WellSpring Pharmaceutical | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| D&C red no. 33 | DIG Info | Solute carrier SLCO2B1 (Ki = 58.1 uM) | [3] | |||
| Sunset yellow FCF | DIG Info | Solute carrier SLCO2B1 (Ki = 68.4 uM) | [3] | |||
| Sodium lauryl sulfate | DIG Info | Solute carrier SLCO2B1 (Ki = 1.98 uM) | [3] | |||
| FD&C red no. 3 | DIG Info | Phosphodiesterase 3A (IC50 = 0.092 uM) | [2] | |||
| Silicon dioxide | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [6] | |||
| Drug Formulation 3 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Lactose; D&c red no. 33; Fd&c yellow no. 6; Fd&c red no. 3; Gelatin
|
|||||
| Dosage Form | Oral Capsule | |||||
| Company | Concordia Pharmaceuticals; Prasco | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| D&C red no. 33 | DIG Info | Solute carrier SLCO2B1 (Ki = 58.1 uM) | [3] | |||
| Sunset yellow FCF | DIG Info | Solute carrier SLCO2B1 (Ki = 68.4 uM) | [3] | |||
| FD&C red no. 3 | DIG Info | Phosphodiesterase 3A (IC50 = 0.092 uM) | [2] | |||
| Drug Formulation 4 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Lactose; Talc
|
|||||
| Dosage Form | Oral Capsule | |||||
| Company | Par Pharmaceutical | |||||
| Phenoxybenzamine Hydrochloride 10mg/capsule capsule | Click to Show/Hide the Full List of Formulation(s): 2 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Alcohol; Ammonia; Butyl Alcohol; D&C Red No. 33; Fd&C Red No. 3; Ferric Oxide Yellow; Gelatin, Unspecified; Isopropyl Alcohol; Lactose Monohydrate; Potassium Hydroxide; Propylene Glycol; Shellac; Silicon Dioxide; Sodium Lauryl Sulfate; Titanium Dioxide
|
|||||
| Dosage Form | Capsule | |||||
| Company | Amneal Pharmaceuticals Ny | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| D&C red no. 33 | DIG Info | Solute carrier SLCO2B1 (Ki = 58.1 uM) | [3] | |||
| Sodium lauryl sulfate | DIG Info | Solute carrier SLCO2B1 (Ki = 1.98 uM) | [3] | |||
| Isopropyl alcohol | DIG Info | Lymphoma P388/ADR cells (IC50 = 0.22 uM) | [4] | |||
| Propylene glycol | DIG Info | Albendazole monooxygenase (Inhibition ratio < 20 %) | [5] | |||
| FD&C red no. 3 | DIG Info | Phosphodiesterase 3A (IC50 = 0.092 uM) | [2] | |||
| Gelatin | DIG Info | Mephenytoin 4-hydroxylase (EC50 = 20.5 uM) | [7] | |||
| Silicon dioxide | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [6] | |||
| Drug Formulation 2 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
D&C Red No. 33; Fd&C Red No. 3; Fd&C Yellow No. 6; Gelatin; Lactose
|
|||||
| Dosage Form | Capsule | |||||
| Company | Concordia Pharmaceuticals ; Prasco Laboratories | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| D&C red no. 33 | DIG Info | Solute carrier SLCO2B1 (Ki = 58.1 uM) | [3] | |||
| Sunset yellow FCF | DIG Info | Solute carrier SLCO2B1 (Ki = 68.4 uM) | [3] | |||
| FD&C red no. 3 | DIG Info | Phosphodiesterase 3A (IC50 = 0.092 uM) | [2] | |||
| Gelatin | DIG Info | Mephenytoin 4-hydroxylase (EC50 = 20.5 uM) | [7] | |||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.
