Details of the Active Pharmaceutical Ingredient (API)
| General Information of API (ID: D00699) | |||||
|---|---|---|---|---|---|
| Name |
Tretinoin
|
||||
| Synonyms |
Click to Show/Hide the Synonyms of This API
Retinoic acid; tretinoin; 302-79-4; all-trans-Retinoic acid; Vitamin A acid; trans-Retinoic acid; Retin-A; Vesanoid; Renova; All-trans Retinoic Acid; all-trans-Vitamin A acid; Dermairol; Aknoten; Aberel; Eudyna; Aknefug; Cordes vas; Epi-aberel; TRETINON; Tretin M; Atralin; all-trans-Vitamin A1 acid; all-trans-Tretinoin; Retionic acid; All Trans Retinoic Acid; Vitamin A1 acid, all-trans-; Retin-A Micro; beta-Retinoic acid; all-(E)-Retinoic acid; Vitamin A acid, all-trans-; Retinoate; Retinoic acid, all-trans-; Alltrans-retinoic acid; Retacnyl; Vesnaroid; NSC-122758; Ro 1-5488; Tretinoin, all-trans-; all trans-Retinoic acid; Retin A; Stieva-A; Tretinoine; Solage; all-trans-beta-Retinoic acid; Effederm; .beta.-Retinoic acid; Tretinoin/All-Trans Retinoic Acid; Aberela [Norway]; Avitoin [Norway]; Effederm [France]; MFCD00001551; UNII-5688UTC01R; A-Acido (Argentina); 3,7-Dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4,6,8-nonatetraenoic acid; MLS000028588; b-Retinoic acid; RETINOIC ACID, ALL TRANS; Tretinoine [INN-French]; Tretinoinum [INN-Latin]; Tretinoina [INN-Spanish]; Tretinoino [INN-Spanish]; CHEMBL38; NSC122758; Atragen; Retinova; SMR000058245; CHEBI:15367; 15-Apo-beta-caroten-15-oic acid; 5688UTC01R; Tretinoin (TN); beta-Ra; Acnavit [Denmark]; AGN 100335; 9-cis-RA; Retin A (TN); NCGC00017280-10; Tretinoinum; Aberela; Acnavit; Avitoin; Betarretin; Tretinoina; Tretinoino; A-Vitaminsyre; all-trans-b-Retinoic acid; DSSTox_CID_1239; Cordes VAS [Germany]; A-Vitaminsyre [Denmark]; DSSTox_RID_76031; DSSTox_GSID_21239; trans-Retinoate; beta-Retinoate; all-trans-Retinoic acid, 97%; tretinoine (French) (EINECS); cis-Retinoic acid; Acide retinoique (French) (DSL); Refissa; Nexret; Vitamin a acid, trans-; Retisol-A; Acid A Vit (Belgium, Netherlands); 3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexenyl)nona-2,4,6,8-tetraenoic acid; 3,7-dimethyl-9-(2,6,6-trimethylcyclohexen-1-yl)nona-2E,4E,6E,8E-tetraenoic acid; (11Z)-retinoic acid; [3H]Retinoic acid; Renova (TN); CCRIS 3294; Avita (TN); HSDB 2169; SR-01000000239; 4759-48-2; EINECS 206-129-0; NSC 122758; BRN 2057223; Tretinoin (JAN/USP/INN); Retinoic acid, cis-9,trans-13-; TNP00194; BML2-E05; [3H]tretinoin; Tretinoin [USAN:USP:INN:BAN]; retinoic acid group; CAS-302-79-4; Prestwick_424; all-(E)-Retinoate; Tretinoine (French); ISORETINOIN; Retinoic acid, cis-; (5E)-Retinoic acid; [3H]Vitamin A acid; PubChem16466; CPD000058245; Retinoic acid all trans; 6-s-trans-retinoic acid; Vitamin-A-sA currencyure; Opera_ID_1055; Prestwick2_000257; Prestwick3_000257; Spectrum5_001746; Spectrum5_001933; acide retinoique (French); Vesanoid (TN) (Roche); Tretinoin - Retinoic Acid; bmse000562; UPCMLD-DP097; R 2625; Renova (0.02% cream); SCHEMBL3145; (9Z,13Z)-Retinoic acid; Altreno (0.05% lotion); BIDD:PXR0081; Lopac0_001061; Avita (0.025% gel); BSPBio_000074; BSPBio_001500; MLS001076515; MLS002207234; MLS002222211; MLS002548861; MLS006010222; ARONIS25153; BIDD:GT0483; SPECTRUM1502016; 9-cis-retinoic acid (9cRA); [3H]RA; BPBio1_000082; cid_444795; GTPL2644; .beta.-all-trans-Retinoic acid; all-trans-retinoic acid (ATRA); DTXSID7021239; SCHEMBL19091395; BDBM31883; HMS502N05; QCR-120; BCPP000036; BDBM323588; HMS1361K22; HMS1568D16; HMS1791K22; HMS1921D14; HMS1989K22; HMS2089D20; HMS2092N11; HMS2095D16; HMS2236N03; HMS3259E11; HMS3263E04; HMS3402K22; HMS3411B09; HMS3675B09; HMS3712D16; Pharmakon1600-01502016; Retinoic acid, all-trans- (8CI); 124510-04-9; ACT00012; BCP01405; US10188615, at-RA; Tox21_110812; Tox21_202330; Tox21_300305; Tox21_501061; All-trans Retinoic Acid (Tretinoin); CCG-39912; LMPR01090019; NSC759631; SBB065722; ZINC12358651; AKOS000280845; Tox21_110812_1; AB03039; AC-6824; CS-1269; DB00755; GS-3578; LP01061; NC00481; NSC-759631; SDCCGSBI-0051031.P004; IDI1_000903; IDI1_033970; NCGC00017280-05; NCGC00017280-06; NCGC00017280-07; NCGC00017280-08; NCGC00017280-09; NCGC00017280-12; NCGC00017280-15; NCGC00017280-16; NCGC00017280-17; NCGC00017280-18; NCGC00017280-19; NCGC00017280-20; NCGC00017280-23; NCGC00017280-38; NCGC00021808-04; NCGC00021808-05; NCGC00021808-06; NCGC00021808-07; NCGC00021808-09; NCGC00021808-11; NCGC00021808-14; NCGC00021808-15; NCGC00254179-01; NCGC00259879-01; NCGC00261746-01; trans-Retinoic acid; Retinoid analogues; BP-20401; BR-73126; HY-14649; Retinoic acid, >=98% (HPLC), powder; ST057075; SBI-0051031.P003; EU-0101061; SW203749-4; 02T794; 5914-EP2275412A1; 5914-EP2292576A2; A10944; C00777; D00094; J10054; Q29417; S-1635; 33998-EP2275420A1; 33998-EP2295055A2; 33998-EP2295416A2; 33998-EP2295426A1; 33998-EP2295427A1; 33998-EP2298748A2; 33998-EP2298764A1; 33998-EP2298765A1; 33998-EP2298768A1; 33998-EP2301928A1; 33998-EP2305642A2; 33998-EP2308833A2; 33998-EP2308861A1; 33998-EP2311453A1; 33998-EP2311808A1; 33998-EP2311829A1; 33998-EP2311840A1; AB00052318-15; AB00052318-16; AB00052318-17; AB00052318_18; AB00052318_19; L000833; Q-200610; SR-01000000239-3; SR-01000000239-4; SR-01000000239-6; SR-01000000239-7; BRD-K06926592-001-01-7; BRD-K71879491-001-15-0; BRD-K71879491-001-22-6; SR-01000000239-12; SR-01000000239-13; SR-01000000239-14; SR-01000000239-15; WLN: L6UTJ A1 B1U1Y1&U2U1Y1&U1VQ C1 C1; Tretinoin, European Pharmacopoeia (EP) Reference Standard; WLN: L6UTJ A1 B1U1Y1 & U2U1Y1 & U1VQ C1 C1; Tretinoin, United States Pharmacopeia (USP) Reference Standard; 3,6,6-trimethyl-1-cyclohexen-1-yl)-2,4,6,8-nonatetraenoic acid; Tretinoin, Pharmaceutical Secondary Standard; Certified Reference Material; 2,4,6,8-Nonatetranoic acid, 3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-; 2,6,8-Nonatetranoic acid, 3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-; 3,7-Dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4,6,8-nonatetraenoate; 97950-17-9
|
||||
| Clinical Status |
Approved
|
||||
| Disease Indication | Acne vulgaris | ICD-11: ED80 | [1] | ||
| PubChem CID | |||||
| Formula |
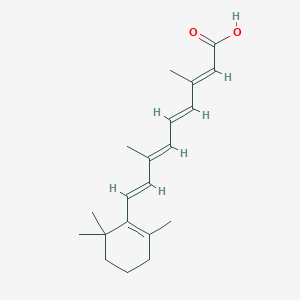
C20H28O2
|
||||
| Canonical SMILES |
CC1=C(C(CCC1)(C)C)/C=C/C(=C/C=C/C(=C/C(=O)O)/C)/C
|
||||
| InChI |
1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+
|
||||
| InChIKey |
SHGAZHPCJJPHSC-YCNIQYBTSA-N
|
||||
| Click to Show/Hide the Molecular Data (Structure/Property) of This API | |||||
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=444795"></iframe>
|
 |
|||
| 3D MOL | 2D MOL | ||||
| Physicochemical Properties | Molecular Weight | 300.4 | Topological Polar Surface Area | 37.3 | |
| XlogP | 6.3 | Complexity | 567 | ||
| Heavy Atom Count | 22 | Rotatable Bond Count | 5 | ||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 2 | ||
| Full List of Drug Formulations (DFMs) Containing This API | ||||||
|---|---|---|---|---|---|---|
| Tretinoin 10 mg capsule | Click to Show/Hide the Full List of Formulation(s): 4 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
D&c yellow no. 10; Fd&c red no. 40; Alpha.-tocopherol; Fd&c blue no. 1; Fd&c blue no. 2; Indigotindisulfonate sodium; Butylated hydroxyanisole; Edetate disodium; Ferric oxide red; Ferric oxide yellow; Ferrosoferric oxide; Propylene glycol; Titanium dioxide; Polysorbate 80; Gelatin; Hydrogenated soybean oil; Shellac; Soybean oil; White wax
|
|||||
| Dosage Form | Oral Capsule | |||||
| Company | Barr Laboratories | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Allura red AC dye | DIG Info | Solute carrier SLCO2B1 (Ki = 4.7 uM) | [2] | |||
| Alpha-tocopherol | DIG Info | Glutathione S-transferase P (IC50 = 500 nM) | [3] | |||
| FD&C blue no. 1 | DIG Info | Solute carrier SLCO2B1 (Ki = 13 uM) | [4] | |||
| FD&C blue no. 2 | DIG Info | Adenosine receptor A3 (IC50 = 1 uM) | [2] | |||
| Quinoline yellow WS | DIG Info | Estrogen receptor alpha (IC50 = 18 uM) | [2] | |||
| Allura red AC dye | DIG Info | Solute carrier SLCO2B1 (Ki = 2.59 uM) | [4] | |||
| Propylene glycol | DIG Info | Albendazole monooxygenase (Inhibition ratio < 20 %) | [5] | |||
| Butylhydroxyanisole | DIG Info | Prostaglandin G/H synthase 2 (IC50 = 2.6 uM) | [2] | |||
| Polysorbate 80 | DIG Info | Prostaglandin G/H synthase 1 (IC50 = 1 uM) | [2] | |||
| Drug Formulation 2 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Ascorbyl palmitate; Oleic acid; Sorbitol; Ammonia; Butylated hydroxyanisole; Edetate disodium; Ferric oxide red; Ferrosoferric oxide; Glycerin; Propylene glycol; Titanium dioxide; Lecithin, soybean; Medium-chain triglycerides; Gelatin; Hydrogenated soybean oil; Shellac; Soybean oil; Sunflower oil; Tocopherol; Yellow wax
|
|||||
| Dosage Form | Oral Capsule | |||||
| Company | Glenmark Pharmaceutials | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Oleic acid | DIG Info | Liver lipid-binding protein (Ki = 180 nM) | [6] | |||
| Ascorbyl palmitate | DIG Info | Thiosulfate sulfurtransferase (IC50 = 53000 nM) | [7] | |||
| Propylene glycol | DIG Info | Albendazole monooxygenase (Inhibition ratio < 20 %) | [5] | |||
| Butylhydroxyanisole | DIG Info | Prostaglandin G/H synthase 2 (IC50 = 2.6 uM) | [2] | |||
| Soybean lecithin | DIG Info | Albendazole monooxygenase (IC50 = 6.61 mg.mL-1) | [5] | |||
| Medium-chain triglyceride | DIG Info | Colon cancer Caco-2 cells (Inhibition ratio > 36 %) | [8] | |||
| Drug Formulation 3 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Butylated hydroxyanisole; Edetate disodium; Ferric oxide red; Ferric oxide yellow; Glycerin; Titanium dioxide; Water; Nitrogen; Lecithin, soybean; Medium-chain triglycerides; Alcohol; Gelatin; Soybean oil; Yellow wax
|
|||||
| Dosage Form | Oral Capsule | |||||
| Company | American Health Packaging; AvKARE; Avera McKennan Hospital | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Butylhydroxyanisole | DIG Info | Prostaglandin G/H synthase 2 (IC50 = 2.6 uM) | [2] | |||
| Soybean lecithin | DIG Info | Albendazole monooxygenase (IC50 = 6.61 mg.mL-1) | [5] | |||
| Medium-chain triglyceride | DIG Info | Colon cancer Caco-2 cells (Inhibition ratio > 36 %) | [8] | |||
| Drug Formulation 4 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Butylated hydroxyanisole; Edetate disodium; Ferric oxide red; Ferric oxide yellow; Glycerin; Titanium dioxide; Nitrogen; Lecithin, soybean; Medium-chain triglycerides; Alcohol; Gelatin; Soybean oil; Yellow wax
|
|||||
| Dosage Form | Oral Capsule | |||||
| Company | Par Pharmaceutical | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Butylhydroxyanisole | DIG Info | Prostaglandin G/H synthase 2 (IC50 = 2.6 uM) | [2] | |||
| Soybean lecithin | DIG Info | Albendazole monooxygenase (IC50 = 6.61 mg.mL-1) | [5] | |||
| Medium-chain triglyceride | DIG Info | Colon cancer Caco-2 cells (Inhibition ratio > 36 %) | [8] | |||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.
