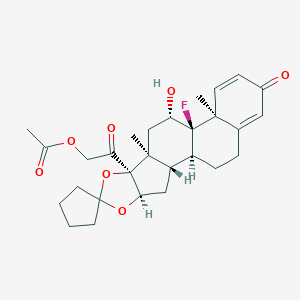
Details of the Active Pharmaceutical Ingredient (API)
| Full List of Drug Formulations (DFMs) Containing This API | ||||||
|---|---|---|---|---|---|---|
| Amcinonide 0.1% ointment | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Benzyl Alcohol; White Petrolatum; Emulsifying Wax; Tenox Ii; Butylated Hydroxyanisole; Propyl Gallate; Citric Acid; Propylene Glycol
|
|||||
| Dosage Form | Ointment | |||||
| Company | Astellas | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Benzyl alcohol | DIG Info | Indoleamine 2,3-dioxygenase 1 (IC50 = 1400 nM) | [1] | |||
| Kyselina citronova | DIG Info | Perilipin-1 (IC50 = 3708 nM) | [2] | |||
| Propyl gallate | DIG Info | Arachidonate 5-lipoxygenase (Ki = 0.43 uM) | [3] | |||
| Propylene glycol | DIG Info | Albendazole monooxygenase (Inhibition ratio < 20 %) | [4] | |||
| Butylhydroxyanisole | DIG Info | Prostaglandin G/H synthase 2 (IC50 = 2.6 uM) | [3] | |||
| Amcinonide 0.1% lotion | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Benzyl Alcohol; Emulsifying Wax; Glycerin; Isopropyl Palmitate; Lactic Acid; Purified Water; Sorbitol Solution; Polyethylene Glycol 400
|
|||||
| Dosage Form | Lotion | |||||
| Company | Astellas | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Benzyl alcohol | DIG Info | Indoleamine 2,3-dioxygenase 1 (IC50 = 1400 nM) | [1] | |||
| Isopropyl palmitate | DIG Info | Acylsphingosine deacylase NAAA (IC50 = 19000 nM) | [5] | |||
| Lactic acid | DIG Info | Fetal lung MRC5 cells (IC50 = 23930 nM) | [6] | |||
| Polyethylene glycol 400 | DIG Info | Albendazole monooxygenase (IC50 = 10.77 mg.mL-1) | [4] | |||
| Amcinonide 0.1% cream | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Benzyl Alcohol; Emulsifying Wax; Glycerin; Isopropyl Palmitate; Lactic Acid; Purified Water; Sorbitol Solution
|
|||||
| Dosage Form | Cream | |||||
| Company | Astellas | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Benzyl alcohol | DIG Info | Indoleamine 2,3-dioxygenase 1 (IC50 = 1400 nM) | [1] | |||
| Isopropyl palmitate | DIG Info | Acylsphingosine deacylase NAAA (IC50 = 19000 nM) | [5] | |||
| Lactic acid | DIG Info | Fetal lung MRC5 cells (IC50 = 23930 nM) | [6] | |||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.