Details of the Active Pharmaceutical Ingredient (API)
| General Information of API (ID: D00654) | |||||
|---|---|---|---|---|---|
| Name |
Telaprevir
|
||||
| Synonyms |
Click to Show/Hide the Synonyms of This API
Telaprevir; 402957-28-2; VX-950; Incivek; Telaprevir (VX-950); MP-424; Incivo; VX 950; Telavic; LY-570310; UNII-655M5O3W0U; VX-950(Telaprevir); MP 424; VRT 111950; VRT-111950; LY 570310; CHEMBL231813; CHEBI:68595; 655M5O3W0U; (1S,3aR,6aS)-2-((S)-2-((S)-2-cyclohexyl-2-(pyrazine-2-carboxamido)acetamido)-3,3-dimethylbutanoyl)-N-((S)-1-(cyclopropylamino)-1,2-dioxohexan-3-yl)octahydrocyclopenta[c]pyrrole-1-carboxamide; (1S,3aR,6aS)-2-((S)-2-((S)-2-cyclohexyl-2-(pyrazine-6-carboxamido)acetamido)-3,3-dimethylbutanoyl)-N-((S)-1-(cyclopropylamino)-1,2-dioxohexan-3-yl)-octahydrocyclopenta[c]pyrrole-1-carboxamide; (3S,3aS,6aR)-2-[(2S)-2-[[(2S)-2-cyclohexyl-2-(pyrazine-2-carbonylamino)acetyl]amino]-3,3-dimethylbutanoyl]-N-[(3S)-1-(cyclopropylamino)-1,2-dioxohexan-3-yl]-3,3a,4,5,6,6a-hexahydro-1H-cyclopenta[c]pyrrole-3-carboxamide; S-Telaprevir; (1S,3aR,6aS)-(2S)-2-cyclohexyl-N-(pyrazinylcarbonyl)glycyl-3-methyl-L-valyl-N-((1S)-1-((cyclopropylamino)oxoacetyl)butyl)octahydrocyclopenta(c)pyrrole-1-carboxamide; (1S,3aR,6aS)-(2S)-2-Cyclohexyl-N-(pyrazinylcarbonyl)glycyl-3-methyl-L-valyl-N-((1S)-1-((cyclopropylamino)oxoacetyl)butyl)octahydrocyclopenta[c]pyrrole-1-carboxamide; Telaprevir [USAN:INN]; HSDB 8125; Incivek(TM); Incivek (TN); Incivo (TN); Telaprevir,VX-950; PubChem22395; Telaprevir (VX950); (1S,3aR,6aS)-2-[(2S)-2-[[(2S)-2-cyclohexyl-2-(pyrazine-2-carbonylamino)acetyl]amino]-3,3-dimethylbutanoyl]-N-[(3S)-1-(cyclopropylamino)-1,2-dioxohexan-3-yl]-3,3a,4,5,6,6a-hexahydro-1H-cyclopenta[c]pyrrole-1-carboxamide; VX-950 (Telaprevir); SCHEMBL183996; Telaprevir (JAN/USAN/INN); GTPL7871; DTXSID40193304; EX-A006; AIDS213006; C36H53N7O6; 569364-34-7; AIDS-213006; AOB87136; ZINC3992480; ABP000273; BDBM50326056; FD7166; MFCD11616089; VRT111950; AKOS005145815; CCG-270366; CS-0285; DB05521; NCGC00346545-03; (3S,3aS,6aR)-2-[(2S)-2-[[(2S)-2-cyclohexyl-2-(pyrazine-2-carbonylamino)acetyl]amino]-3,3-dimethyl-butanoyl]-N-[(1S)-1-[2-(cyclopropylamino)-2-oxo-acetyl]butyl]-3,3a,4,5,6,6a-hexahydro-1H-cyclopenta[c]pyrrole-3-carboxamide; AS-16995; HY-10235; AB0027979; WO-00218369; A18739; D09012; Q-4436; 957T282; Q408557; Q-101417; (1S,3aR,6aS)-(2S)-2-Cyclohexyl-N-(2-pyrazinylcarbonyl)glycyl-3-methyl-L-valyl-N-[(1S)-1-[2-(cyclopropylamino)-2-oxoacetyl]butyl]octahydrocyclopenta[c]pyrrole-1-carboxamide; (1S,3aR,6aS)-2-((S)-2-((S)-2-cyclohexyl-2-(pyrazine-2-carboxamido)acetamido)-3,3-dimethylbutanoyl)-N-((S)-1-(cyclopropylamino)-1,2-dioxo-hexan-3-yl)octahydrocyclopenta[c]pyrrole-1-carboxamide; (1S,3aR,6aS)-2-((S)-2-((S)-2-cyclohexyl-2-(pyrazine-2-carboxamido)acetamido)-3,3-dimethylbutanoyl)-N-((S)-1-(cydopropylamino)-1,2-dioxo-hexan-3-yl)octahydrocyclopenta[c]pyrrole-1-carboxamide; (1S,3aR,6aS)-2-((S)-2-((S)-2-cyclohexyl-2-(pyrazine-3-carboxamido)acetamido)-3,3-dimethylbutanoyl)-N-((S)-1-(cyclopropylamino)-1,2-dioxohexan-3-yl)-octahydrocyclopenta[c]pyrrole-1-carboxamide; (1S,3aR,6aS)-2-[(2S)-2-({(2S)-2-cyclohexyl-2-[(pyrazin-2-ylcarbonyl)amino]acetyl}amino)-3,3-dimethylbutanoyl]-N-[(3S)-1-(cyclopropylamino)-1,2-dioxohexan-3-yl]octahydrocyclopenta[c]pyrrole-1-carboxamide; (1S,3aR,6aS)-N-((S)-2-((S)-2-cyclohexyl-2-(pyrazine-2-carboxamido)acetamido)-3,3-dimethylbutanoyl)-N-((S)-1-(cyclopropylamino)-1,2-dioxohexan-3-yl)octahydrocyclopenta[c]pyrrole-1-carboxamide; (3S)-3-{[(1S,3aR,6aS)-2-[(2S)-2-[(2S)-2-cyclohexyl-2-(pyrazin-2-ylformamido)acetamido]-3,3-dimethylbutanoyl]-octahydrocyclopenta[c]pyrrol-1-yl]formamido}-N-cyclopropyl-2-oxohexanamide; 2-(2-{2-Cyclohexyl-2-[(pyrazine-2-carbonyl)-amino]-acetylamino}-3,3-dimethyl-butyryl)-octahydro-cyclopenta[c]pyrrole-1-carboxylic acid (1-cyclopropylaminooxalyl-butyl)-amide; Cyclopenta(c)pyrrole-1-carboxamide, (2S)-2-cyclohexyl-N-(pyrazinylcarbonyl)glycyl-3-methyl-L-valyl-N-((1S)-1-((cyclopropylamino)oxoacetyl)butyl)octahydro-, (1S,3aR,6aS)-
|
||||
| Clinical Status |
Approved
|
||||
| Disease Indication | Viral hepatitis | ICD-11: 1E51 | [1] | ||
| PubChem CID | |||||
| Formula |
C36H53N7O6
|
||||
| Canonical SMILES |
CCC[C@@H](C(=O)C(=O)NC1CC1)NC(=O)[C@@H]2[C@H]3CCC[C@H]3CN2C(=O)[C@H](C(C)(C)C)NC(=O)[C@H](C4CCCCC4)NC(=O)C5=NC=CN=C5
|
||||
| InChI |
1S/C36H53N7O6/c1-5-10-25(29(44)34(48)39-23-15-16-23)40-33(47)28-24-14-9-13-22(24)20-43(28)35(49)30(36(2,3)4)42-32(46)27(21-11-7-6-8-12-21)41-31(45)26-19-37-17-18-38-26/h17-19,21-25,27-28,30H,5-16,20H2,1-4H3,(H,39,48)(H,40,47)(H,41,45)(H,42,46)/t22-,24-,25-,27-,28-,30+/m0/s1
|
||||
| InChIKey |
BBAWEDCPNXPBQM-GDEBMMAJSA-N
|
||||
| Click to Show/Hide the Molecular Data (Structure/Property) of This API | |||||
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=3010818"></iframe>
|
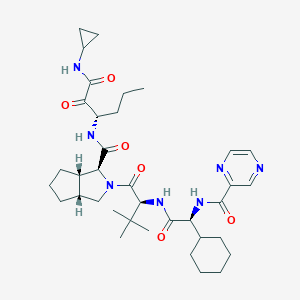 |
|||
| 3D MOL is unavailable | 2D MOL | ||||
| Physicochemical Properties | Molecular Weight | 679.8 | Topological Polar Surface Area | 180 | |
| XlogP | 4.2 | Complexity | 1240 | ||
| Heavy Atom Count | 49 | Rotatable Bond Count | 14 | ||
| Hydrogen Bond Donor Count | 4 | Hydrogen Bond Acceptor Count | 8 | ||
| Full List of Drug Formulations (DFMs) Containing This API | ||||||
|---|---|---|---|---|---|---|
| Telaprevir 375 mg tablet | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Sodium lauryl sulfate; Fd&c red no. 40; Sodium stearyl fumarate; Fd&c blue no. 2; Talc; Titanium dioxide; Croscarmellose sodium; Polyethylene glycol 3350; Polyvinyl alcohol; Silicon dioxide; Calcium phosphate, dibasic, anhydrous; Cellulose, microcrystalline; Hypromellose acetate succinate 06081224 (3 mm2/s)
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | Vertex Pharmaceuticals | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Allura red AC dye | DIG Info | Solute carrier SLCO2B1 (Ki = 4.7 uM) | [2] | |||
| Sodium lauryl sulfate | DIG Info | Solute carrier SLCO2B1 (Ki = 1.98 uM) | [3] | |||
| FD&C blue no. 2 | DIG Info | Adenosine receptor A3 (IC50 = 1 uM) | [2] | |||
| Allura red AC dye | DIG Info | Solute carrier SLCO2B1 (Ki = 2.59 uM) | [3] | |||
| Sodium stearyl fumarate | DIG Info | Leukemia K562 cells (IC50 = 20.2 ug.mL-1) | [4] | |||
| Polyvinyl alcohol | DIG Info | Debrisoquine 4-hydroxylase (EC50 = 354.8 uM) | [5] | |||
| Carmellose sodium | DIG Info | Albendazole monooxygenase (Protein expression upregulation) | [6] | |||
| Polyethylene glycol 3350 | DIG Info | Albendazole monooxygenase (Protein expression upregulation) | [6] | |||
| Silicon dioxide | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [6] | |||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.
