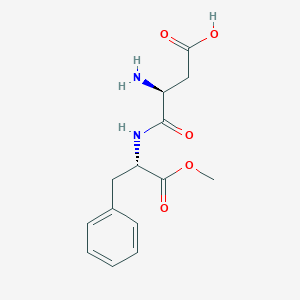
Details of the Drug Inactive Ingredient (DIG)
| Full List of Active Pharmaceutical Ingredients (APIs) Co-administrated with This DIG | ||||||
|---|---|---|---|---|---|---|
| ICD Disease Classification 01 | Infectious/parasitic disease | Click to Show/Hide | ||||
|
Didanosine
|
API Info |
Human immunodeficiency virus infection [ICD-11: 1C60]
|
[1] | |||
|
Raltegravir
|
API Info |
Human immunodeficiency virus infection [ICD-11: 1C60]
|
[2] | |||
|
Cefixime
|
API Info |
Streptococcus bacterial infection [ICD-11: 1C41]
|
[3] | |||
| ICD Disease Classification 04 | Immune system disease | Click to Show/Hide | ||||
|
Cetirizine
|
API Info |
Allergy [ICD-11: 4A80]
|
[4] | |||
| ICD Disease Classification 06 | Mental/behavioural/neurodevelopmental disorder | Click to Show/Hide | ||||
|
Risperidone
|
API Info |
Schizophrenia [ICD-11: 6A20]
|
[5] | |||
|
Alprazolam
|
API Info |
Anxiety disorder [ICD-11: 6B00]
|
[6] | |||
|
Lamotrigine
|
API Info |
Bipolar disorder [ICD-11: 6A60]
|
[7] | |||
|
Olanzapine
|
API Info |
Schizophrenia [ICD-11: 6A20]
|
[8] | |||
|
Aripiprazole
|
API Info |
Schizophrenia [ICD-11: 6A20]
|
[9] | |||
|
Methylphenidate
|
API Info |
Attention deficit hyperactivity disorder [ICD-11: 6A05]
|
[10] | |||
|
Mirtazapine
|
API Info |
Major depressive disorder [ICD-11: 6A70]
|
[11] | |||
|
Clozapine
|
API Info |
Schizophrenia [ICD-11: 6A20]
|
[12] | |||
| ICD Disease Classification 07 | Sleep-wake disorder | Click to Show/Hide | ||||
|
Zolpidem
|
API Info |
Insomnia [ICD-11: 7A00]
|
[13] | |||
| ICD Disease Classification 08 | Nervous system disease | Click to Show/Hide | ||||
|
Acetaminophen
|
API Info |
Anaesthesia [ICD-11: 8E22]
|
[14] | |||
|
Tramadol
|
API Info |
Anaesthesia [ICD-11: 8E22]
|
[15] | |||
|
Clonazepam
|
API Info |
Epilepsy [ICD-11: 8A60]
|
[16] | |||
|
Rizatriptan
|
API Info |
Migraine [ICD-11: 8A80]
|
[17] | |||
|
Donepezil
|
API Info |
Alzheimer disease [ICD-11: 8A20]
|
[18] | |||
|
Zolmitriptan
|
API Info |
Migraine [ICD-11: 8A80]
|
[19] | |||
|
Selegiline
|
API Info |
Parkinsonism [ICD-11: 8A00]
|
[20] | |||
| ICD Disease Classification 10 | Ear/mastoid process disease | Click to Show/Hide | ||||
|
Amoxicillin
|
API Info |
Acute otitis media [ICD-11: AB00]
|
[21] | |||
| ICD Disease Classification 11 | Circulatory system disease | Click to Show/Hide | ||||
|
Bosentan
|
API Info |
Pulmonary hypertension [ICD-11: BB01]
|
[22] | |||
| ICD Disease Classification 12 | Respiratory system disease | Click to Show/Hide | ||||
|
Montelukast
|
API Info |
Asthma [ICD-11: CA23]
|
[23] | |||
|
Guaifenesin
|
API Info |
Asthma [ICD-11: CA23]
|
[24] | |||
|
Fexofenadine
|
API Info |
Allergic rhinitis [ICD-11: CA08]
|
[25] | |||
|
Loratadine
|
API Info |
Allergic rhinitis [ICD-11: CA08]
|
[26] | |||
|
Desloratadine
|
API Info |
Allergic rhinitis [ICD-11: CA08]
|
[27] | |||
| ICD Disease Classification 13 | Digestive system disease | Click to Show/Hide | ||||
|
Meclizine
|
API Info |
Functional nausea/vomiting [ICD-11: DD90]
|
[28] | |||
|
Lansoprazole
|
API Info |
Duodenal ulcer [ICD-11: DA63]
|
[29] | |||
|
Bismuth subsalicylate
|
API Info |
Irritable bowel syndrome [ICD-11: DD91]
|
[30] | |||
|
Ondansetron
|
API Info |
Gastritis [ICD-11: DA42]
|
[31] | |||
|
Metoclopramide
|
API Info |
Gastro-oesophageal reflux disease [ICD-11: DA22]
|
[32] | |||
|
Mesalamine
|
API Info |
Inflammatory bowel disease [ICD-11: DD72]
|
[33] | |||
|
Dimenhydrinate
|
API Info |
Functional nausea/vomiting [ICD-11: DD90]
|
[34] | |||
| ICD Disease Classification 16 | Genitourinary system disease | Click to Show/Hide | ||||
|
Ibuprofen
|
API Info |
Dysmenorrhea [ICD-11: GA34]
|
[35] | |||
| ICD Disease Classification 17 | Sexual health-related condition | Click to Show/Hide | ||||
|
Vardenafil
|
API Info |
Erectile dysfunction [ICD-11: HA01]
|
[36] | |||
| Full List of Biological Targets of DIG (DBTs) Regulated by This DIG | ||||||
|---|---|---|---|---|---|---|
| Hydrolase (HDase) | ||||||
| DBT Name: Matrix metalloproteinase-1 (MMP1) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | IC50 = 20000 nM (estimated based on the structural similarity with CHEMBL62186 ) | [37] | ||||
| Structural Similarity | Tanimoto coefficient = 0.916666667 | |||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | MMP1_HUMAN | |||||
| Nuclear receptor (NR) | ||||||
| DBT Name: Pregnane X receptor (NR1I2) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | Ki = 13 uM (tested by experiment) | [38] | ||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | NR1I2_HUMAN | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.