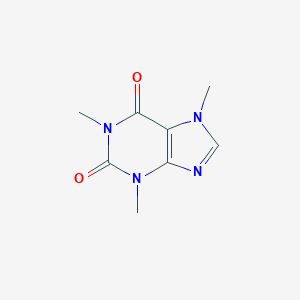
Details of the Drug Inactive Ingredient (DIG)
| Full List of Biological Targets of DIG (DBTs) Regulated by This DIG | ||||||
|---|---|---|---|---|---|---|
| G-protein coupled receptor (GPCR) | ||||||
| DBT Name: Adenosine receptor (ADOR) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | Ki = 15000 nM (tested by experiment) | [1] | ||||
| Tested Species | Rattus norvegicus (Rat) | |||||
| UniProt ID | AA1R_RAT ; AA2AR_RAT ; AA2BR_RAT ; AA3R_RAT | |||||
| DBT Name: Adenosine receptor A1 (AA1R) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment (1) for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | Ki > 10000 nM (tested by experiment) | [2] | ||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | AA1R_HUMAN | |||||
| Experiment (2) for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | Ki = 130000 nM (tested by experiment) | [1] | ||||
| Tested Species | Bos taurus (Bovine) | |||||
| UniProt ID | AA1R_BOVIN | |||||
| Experiment (3) for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | Ki = 100000 nM (tested by experiment) | [3] | ||||
| Tested Species | Cavia porcellus (Guinea pig) | |||||
| UniProt ID | AA1R_CAVPO | |||||
| Experiment (4) for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | Ki > 10000 nM (tested by experiment) | [2] | ||||
| Tested Species | Rattus norvegicus (Rat) | |||||
| UniProt ID | AA1R_RAT | |||||
| DBT Name: Adenosine receptor A2 (ADORA2) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | Ki = 27000 nM (tested by experiment) | [4] | ||||
| Tested Species | Rattus norvegicus (Rat) | |||||
| UniProt ID | AA2AR_RAT ; AA2BR_RAT | |||||
| DBT Name: Adenosine receptor A2a (AA2AR) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment (1) for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | Ki = 2480 nM (tested by experiment) | [5] | ||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | AA2AR_HUMAN | |||||
| Experiment (2) for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | Ki = 50000 nM (tested by experiment) | [6] | ||||
| Tested Species | Cavia porcellus (Guinea pig) | |||||
| UniProt ID | AA2AR_CAVPO | |||||
| Experiment (3) for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | Ki = 9400 nM (tested by experiment) | [7] | ||||
| Tested Species | Rattus norvegicus (Rat) | |||||
| UniProt ID | AA2AR_RAT | |||||
| DBT Name: Adenosine receptor A2b (AA2BR) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment (1) for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | Ki = 10000 nM (tested by experiment) | [8] | ||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | AA2BR_HUMAN | |||||
| Experiment (2) for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | Ki = 23000 nM (tested by experiment) | [2] | ||||
| Tested Species | Mus musculus (Mouse) | |||||
| UniProt ID | AA2BR_MOUSE | |||||
| Experiment (3) for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | Ki = 30000 nM (tested by experiment) | [2] | ||||
| Tested Species | Rattus norvegicus (Rat) | |||||
| UniProt ID | AA2BR_RAT | |||||
| DBT Name: Adenosine receptor A3 (AA3R) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment (1) for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | Ki = 13300 nM (tested by experiment) | [9] | ||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | AA3R_HUMAN | |||||
| Experiment (2) for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | Ki > 100000 nM (tested by experiment) | [10] | ||||
| Tested Species | Rattus norvegicus (Rat) | |||||
| UniProt ID | AA3R_RAT | |||||
| Oxidoreductase (ORase) | ||||||
| DBT Name: Monoamine oxidase A (MAO-A) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | IC50 > 50000 nM (tested by experiment) | [11] | ||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | AOFA_HUMAN | |||||
| DBT Name: Monoamine oxidase B (MAO-B) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | IC50 > 50000 nM (tested by experiment) | [11] | ||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | AOFB_HUMAN | |||||
| Transferase (TFase) | ||||||
| DBT Name: Myophosphorylase (PYGM) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment (1) for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | IC50 = 114000 nM (tested by experiment) | [12] | ||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | PYGM_HUMAN | |||||
| Experiment (2) for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | IC50 = 74900 nM (tested by experiment) | [13] | ||||
| Tested Species | Oryctolagus cuniculus (Rabbit) | |||||
| UniProt ID | PYGM_RABIT | |||||
| Experiment (3) for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | IC50 = 182000 nM (tested by experiment) | [14] | ||||
| Tested Species | Rattus norvegicus (Rat) | |||||
| UniProt ID | PYGM_RAT | |||||
| DBT Name: PI3-kinase alpha (PIK3CA) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | IC50 = 400000 nM (tested by experiment) | [15] | ||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | PK3CA_HUMAN | |||||
| DBT Name: PI3-kinase beta (PIK3CB) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | IC50 = 400000 nM (tested by experiment) | [15] | ||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | PK3CB_HUMAN | |||||
| DBT Name: PI3-kinase delta (PIK3CD) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | IC50 = 75000 nM (tested by experiment) | [15] | ||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | PK3CD_HUMAN | |||||
| Hydrolase (HDase) | ||||||
| DBT Name: Acetylcholinesterase (ACHE) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | IC50 = 7250 nM (tested by experiment) | [16] | ||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | ACES_HUMAN | |||||
| DBT Name: Cholinesterase (BCHE) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | IC50 > 50000 nM (tested by experiment) | [16] | ||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | CHLE_HUMAN | |||||
| DBT Name: Phosphodiesterase 1 (PDE1) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | IC50 = 70000 nM (tested by experiment) | [17] | ||||
| Tested Species | Rattus norvegicus (Rat) | |||||
| UniProt ID | PDE1B_RAT ; PDE1C_RAT | |||||
| Primary active transporter (PAT) | ||||||
| DBT Name: Multidrug resistance protein 3 (ABCC3) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | IC50 = 133000 nM (tested by experiment) | [18] | ||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | MRP3_HUMAN | |||||
| DBT Name: Bile salt export pump (BSEP) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | IC50 = 133000 nM (tested by experiment) | [18] | ||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | ABCBB_HUMAN | |||||
| DBT Name: Multidrug resistance protein 4 (ABCC4) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | IC50 = 133000 nM (tested by experiment) | [18] | ||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | MRP4_HUMAN | |||||
| DBT Name: Multidrug resistance protein 2 (ABCC2) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | IC50 = 133000 nM (tested by experiment) | [18] | ||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | MRP2_HUMAN | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.