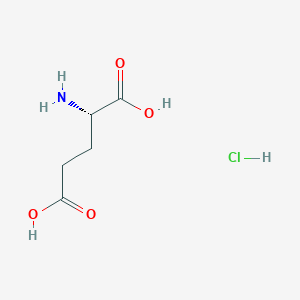
Details of the Drug Inactive Ingredient (DIG)
| Full List of Active Pharmaceutical Ingredients (APIs) Co-administrated with This DIG | ||||||
|---|---|---|---|---|---|---|
| ICD Disease Classification 06 | Mental/behavioural/neurodevelopmental disorder | Click to Show/Hide | ||||
|
Bupropion
|
API Info |
Nicotine dependence [ICD-11: 6C4A]
|
[1] | |||
| Full List of Biological Targets of DIG (DBTs) Regulated by This DIG | ||||||
|---|---|---|---|---|---|---|
| G-protein coupled receptor (GPCR) | ||||||
| DBT Name: Glutamate receptor mGLU1 (mGluR1) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment (1) for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | Ki = 250 nM (estimated based on the structural similarity with CHEMBL575060 ) | [2] | ||||
| Structural Similarity | Tanimoto coefficient = 1 | |||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | GRM1_HUMAN | |||||
| Experiment (2) for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | Ki = 340 nM (estimated based on the structural similarity with CHEMBL575060 ) | [3] | ||||
| Structural Similarity | Tanimoto coefficient = 1 | |||||
| Tested Species | Rattus norvegicus (Rat) | |||||
| UniProt ID | GRM1_RAT | |||||
| DBT Name: Glutamate receptor mGLU2 (mGluR2) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | EC50 = 290 nM (estimated based on the structural similarity with CHEMBL575060 ) | [4] | ||||
| Structural Similarity | Tanimoto coefficient = 0.979166667 | |||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | GRM2_HUMAN | |||||
| DBT Name: Glutamate receptor mGLU3 (mGluR3) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | Ki = 57.54 nM (estimated based on the structural similarity with CHEMBL575060 ) | [5] | ||||
| Structural Similarity | Tanimoto coefficient = 1 | |||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | GRM3_HUMAN | |||||
| DBT Name: Glutamate receptor mGLU4 (mGluR4) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | Ki = 1400 nM (estimated based on the structural similarity with CHEMBL575060 ) | [6] | ||||
| Structural Similarity | Tanimoto coefficient = 1 | |||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | GRM4_HUMAN | |||||
| DBT Name: Glutamate receptor mGLU5 (mGluR5) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | Ki = 390 nM (estimated based on the structural similarity with CHEMBL575060 ) | [2] | ||||
| Structural Similarity | Tanimoto coefficient = 1 | |||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | GRM5_HUMAN | |||||
| DBT Name: Glutamate receptor mGLU6 (mGluR6) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | EC50 = 4900 nM (estimated based on the structural similarity with CHEMBL575060 ) | [4] | ||||
| Structural Similarity | Tanimoto coefficient = 0.979166667 | |||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | GRM6_HUMAN | |||||
| DBT Name: Glutamate receptor mGLU7 (mGluR7) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | EC50 > 100000 nM (estimated based on the structural similarity with CHEMBL575060 ) | [7] | ||||
| Structural Similarity | Tanimoto coefficient = 1 | |||||
| Tested Species | Rattus norvegicus (Rat) | |||||
| UniProt ID | GRM7_RAT | |||||
| DBT Name: Glutamate receptor mGLU8 (mGluR8) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment (1) for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | IC50 = 5.7 nM (estimated based on the structural similarity with CHEMBL575060 ) | [8] | ||||
| Structural Similarity | Tanimoto coefficient = 1 | |||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | GRM8_HUMAN | |||||
| Experiment (2) for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | Ki = 3400 nM (estimated based on the structural similarity with CHEMBL575060 ) | [9] | ||||
| Structural Similarity | Tanimoto coefficient = 1 | |||||
| Tested Species | Rattus norvegicus (Rat) | |||||
| UniProt ID | GRM8_RAT | |||||
| Oxidoreductase (ORase) | ||||||
| DBT Name: Glutathione reductase (GSR) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | Ki = 42700 nM (estimated based on the structural similarity with CHEMBL575060 ) | [10] | ||||
| Structural Similarity | Tanimoto coefficient = 1 | |||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | GSHR_HUMAN | |||||
| Transferase (TFase) | ||||||
| DBT Name: ATP-citrate synthase (ACLY) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | Ki = 300000 nM (estimated based on the structural similarity with CHEMBL575060 ) | [11] | ||||
| Structural Similarity | Tanimoto coefficient = 1 | |||||
| Tested Species | Rattus norvegicus (Rat) | |||||
| UniProt ID | ACLY_RAT | |||||
| Hydrolase (HDase) | ||||||
| DBT Name: Glutamate carboxypeptidase II (FOLH1) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | IC50 = 428000 nM (estimated based on the structural similarity with CHEMBL575060 ) | [12] | ||||
| Structural Similarity | Tanimoto coefficient = 1 | |||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | FOLH1_HUMAN | |||||
| Lyase/isomerase/ligase (L/I/G) | ||||||
| DBT Name: Cytochrome P450 2J2 (CYP2J2) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | Ki = 51 nM (estimated based on the structural similarity with CHEMBL575060 ) | [13] | ||||
| Structural Similarity | Tanimoto coefficient = 1 | |||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | EAA3_HUMAN | |||||
| Potential-driven transporter (PDT) | ||||||
| DBT Name: Glutamate/aspartate transporter 1 (GLAST1) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | IC50 = 11000 nM (estimated based on the structural similarity with CHEMBL575060 ) | [14] | ||||
| Structural Similarity | Tanimoto coefficient = 1 | |||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | EAA1_HUMAN | |||||
| DBT Name: Glutamate/aspartate transporter 2 (GLT1) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | IC50 = 52000 nM (estimated based on the structural similarity with CHEMBL575060 ) | [14] | ||||
| Structural Similarity | Tanimoto coefficient = 1 | |||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | EAA2_HUMAN | |||||
| DBT Name: Excitatory amino-acid carrier 1 (EAAC1) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | Ki = 51 nM (estimated based on the structural similarity with CHEMBL575060 ) | [13] | ||||
| Structural Similarity | Tanimoto coefficient = 1 | |||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | EAA3_HUMAN | |||||
| DBT Name: Solute carrier SLC1A6 (EAAT4) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | IC50 = 13000 nM (estimated based on the structural similarity with CHEMBL575060 ) | [14] | ||||
| Structural Similarity | Tanimoto coefficient = 1 | |||||
| Tested Species | Rattus norvegicus (Rat) | |||||
| UniProt ID | EAA4_RAT | |||||
| Transmembrane channel/porin (TC/P) | ||||||
| DBT Name: Glutamate receptor NMDA (GRIN) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment (1) for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | IC50 = 70 nM (estimated based on the structural similarity with CHEMBL575060 ) | [15] | ||||
| Structural Similarity | Tanimoto coefficient = 1 | |||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | NMDZ1_HUMAN ; NMDE1_HUMAN ; NMDE2_HUMAN ; NMDE3_HUMAN ; NMDE4_HUMAN ; NMD3A_HUMAN ; NMD3B_HUMAN | |||||
| Experiment (2) for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | IC50 = 69 nM (estimated based on the structural similarity with CHEMBL575060 ) | [16] | ||||
| Structural Similarity | Tanimoto coefficient = 1 | |||||
| Tested Species | Rattus norvegicus (Rat) | |||||
| UniProt ID | NMDZ1_RAT ; NMDE1_RAT ; NMDE2_RAT ; NMDE3_RAT ; NMDE4_RAT ; NMD3A_RAT ; NMD3B_RAT | |||||
| DBT Name: GluA1-GluA2 complex (GRIA1-GRIA2) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | EC50 = 6160 nM (estimated based on the structural similarity with CHEMBL575060 ) | [17] | ||||
| Structural Similarity | Tanimoto coefficient = 0.979166667 | |||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | GRIA1_HUMAN ; GRIA2_HUMAN | |||||
| DBT Name: Glutamate receptor AMPA 3 (GRIA3) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | Ki = 249 nM (estimated based on the structural similarity with CHEMBL575060 ) | [18] | ||||
| Structural Similarity | Tanimoto coefficient = 1 | |||||
| Tested Species | Rattus norvegicus (Rat) | |||||
| UniProt ID | GRIA3_RAT | |||||
| DBT Name: Glutamate receptor AMPA 4 (GRIA4) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | Ki = 354 nM (estimated based on the structural similarity with CHEMBL575060 ) | [18] | ||||
| Structural Similarity | Tanimoto coefficient = 1 | |||||
| Tested Species | Rattus norvegicus (Rat) | |||||
| UniProt ID | GRIA4_RAT | |||||
| DBT Name: Glutamate receptor AMPA 1 (GRIA1) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment (1) for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | Ki = 1360 nM (estimated based on the structural similarity with CHEMBL575060 ) | [19] | ||||
| Structural Similarity | Tanimoto coefficient = 1 | |||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | GRIA1_HUMAN | |||||
| Experiment (2) for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | Ki = 169 nM (estimated based on the structural similarity with CHEMBL575060 ) | [18] | ||||
| Structural Similarity | Tanimoto coefficient = 1 | |||||
| Tested Species | Rattus norvegicus (Rat) | |||||
| UniProt ID | GRIA1_RAT | |||||
| DBT Name: Glutamate receptor AMPA 2 (GRIA2) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment (1) for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | Ki = 940 nM (estimated based on the structural similarity with CHEMBL575060 ) | [19] | ||||
| Structural Similarity | Tanimoto coefficient = 1 | |||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | GRIA2_HUMAN | |||||
| Experiment (2) for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | Ki = 280 nM (estimated based on the structural similarity with CHEMBL575060 ) | [20] | ||||
| Structural Similarity | Tanimoto coefficient = 1 | |||||
| Tested Species | Rattus norvegicus (Rat) | |||||
| UniProt ID | GRIA2_RAT | |||||
| DBT Name: Glutamate receptor kainate 5 (GRIK5) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | Ki = 701 nM (estimated based on the structural similarity with CHEMBL575060 ) | [19] | ||||
| Structural Similarity | Tanimoto coefficient = 1 | |||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | GRIK5_HUMAN | |||||
| DBT Name: Glutamate receptor (GRIA) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | IC50 = 613 nM (estimated based on the structural similarity with CHEMBL575060 ) | [21] | ||||
| Structural Similarity | Tanimoto coefficient = 1 | |||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | GRIA1_HUMAN ; GRIA2_HUMAN ; GRIA3_HUMAN ; GRIA4_HUMAN | |||||
| DBT Name: Glutamate receptor kainate 1 (GRIK1) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | Ki = 138.04 nM (estimated based on the structural similarity with CHEMBL575060 ) | [22] | ||||
| Structural Similarity | Tanimoto coefficient = 1 | |||||
| Tested Species | Rattus norvegicus (Rat) | |||||
| UniProt ID | GRIK1_RAT | |||||
| DBT Name: Glutamate receptor kainate 2 (GRIK2) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment (1) for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | Ki = 1106 nM (estimated based on the structural similarity with CHEMBL575060 ) | [23] | ||||
| Structural Similarity | Tanimoto coefficient = 1 | |||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | GRIK2_HUMAN | |||||
| Experiment (2) for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | Ki = 330 nM (estimated based on the structural similarity with CHEMBL575060 ) | [20] | ||||
| Structural Similarity | Tanimoto coefficient = 1 | |||||
| Tested Species | Rattus norvegicus (Rat) | |||||
| UniProt ID | GRIK2_RAT | |||||
| DBT Name: Glutamate receptor kainate 3 (GRIK3) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | Ki = 489.78 nM (estimated based on the structural similarity with CHEMBL575060 ) | [22] | ||||
| Structural Similarity | Tanimoto coefficient = 1 | |||||
| Tested Species | Rattus norvegicus (Rat) | |||||
| UniProt ID | GRIK3_RAT | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.