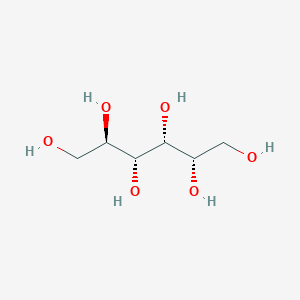
Details of the Drug Inactive Ingredient (DIG)
| Full List of Active Pharmaceutical Ingredients (APIs) Co-administrated with This DIG | ||||||
|---|---|---|---|---|---|---|
| ICD Disease Classification 01 | Infectious/parasitic disease | Click to Show/Hide | ||||
|
Amantadine
|
API Info |
Influenza virus infection [ICD-11: 1E30]
|
[1] | |||
|
Didanosine
|
API Info |
Human immunodeficiency virus infection [ICD-11: 1C60]
|
[2] | |||
|
Ethambutol
|
API Info |
Pulmonary tuberculosis [ICD-11: 1B10]
|
[3] | |||
|
Raltegravir
|
API Info |
Human immunodeficiency virus infection [ICD-11: 1C60]
|
[4] | |||
| ICD Disease Classification 02 | Benign/in-situ/malignant neoplasm | Click to Show/Hide | ||||
|
Bexarotene
|
API Info |
Anaplastic large cell lymphoma [ICD-11: 2A90]
|
[5] | |||
| ICD Disease Classification 04 | Immune system disease | Click to Show/Hide | ||||
|
Cetirizine
|
API Info |
Allergy [ICD-11: 4A80]
|
[6] | |||
| ICD Disease Classification 05 | Endocrine/nutritional/metabolic disease | Click to Show/Hide | ||||
|
Paricalcitol
|
API Info |
Hyperparathyroidism [ICD-11: 5A51]
|
[7] | |||
|
Benzphetamine
|
API Info |
Obesity [ICD-11: 5B81]
|
[8] | |||
|
Calcifediol
|
API Info |
Vitamin deficiency [ICD-11: 5B55]
|
[9] | |||
|
Omega-3 carboxylic acid
|
API Info |
Hypertriglyceridaemia [ICD-11: 5C80]
|
[10] | |||
| ICD Disease Classification 06 | Mental/behavioural/neurodevelopmental disorder | Click to Show/Hide | ||||
|
Risperidone
|
API Info |
Schizophrenia [ICD-11: 6A20]
|
[11] | |||
|
Alprazolam
|
API Info |
Anxiety disorder [ICD-11: 6B00]
|
[12] | |||
|
Lithium carbonate
|
API Info |
Bipolar disorder [ICD-11: 6A60]
|
[13] | |||
| ICD Disease Classification 07 | Sleep-wake disorder | Click to Show/Hide | ||||
|
Zolpidem
|
API Info |
Insomnia [ICD-11: 7A00]
|
[14] | |||
| ICD Disease Classification 08 | Nervous system disease | Click to Show/Hide | ||||
|
Acetaminophen
|
API Info |
Anaesthesia [ICD-11: 8E22]
|
[15] | |||
|
Carbamazepine
|
API Info |
Epilepsy [ICD-11: 8A60]
|
[16] | |||
|
Clonazepam
|
API Info |
Epilepsy [ICD-11: 8A60]
|
[17] | |||
|
Rizatriptan
|
API Info |
Migraine [ICD-11: 8A80]
|
[18] | |||
|
Ethosuximide
|
API Info |
Epilepsy [ICD-11: 8A60]
|
[19] | |||
| ICD Disease Classification 09 | Visual system disease | Click to Show/Hide | ||||
|
Dextromethorphan
|
API Info |
Allergic conjunctivitis [ICD-11: 9A60]
|
[20] | |||
| ICD Disease Classification 10 | Ear/mastoid process disease | Click to Show/Hide | ||||
|
Diphenhydramine
|
API Info |
Meniere disease [ICD-11: AB31]
|
[21] | |||
| ICD Disease Classification 11 | Circulatory system disease | Click to Show/Hide | ||||
|
Aspirin
|
API Info |
Myocardial infarction [ICD-11: BA41]
|
[22] | |||
|
Nifedipine
|
API Info |
Angina pectoris [ICD-11: BA40]
|
[23] | |||
|
Telmisartan
|
API Info |
Essential hypertension [ICD-11: BA00]
|
[24] | |||
|
Nimodipine
|
API Info |
Cerebral vasospasm [ICD-11: BA85]
|
[25] | |||
|
Icosapent ethyl
|
API Info |
Myocardial infarction [ICD-11: BA41]
|
[26] | |||
| ICD Disease Classification 12 | Respiratory system disease | Click to Show/Hide | ||||
|
Benzonatate
|
API Info |
Asthma [ICD-11: CA23]
|
[27] | |||
|
Loratadine
|
API Info |
Allergic rhinitis [ICD-11: CA08]
|
[28] | |||
| ICD Disease Classification 13 | Digestive system disease | Click to Show/Hide | ||||
|
Lansoprazole
|
API Info |
Duodenal ulcer [ICD-11: DA63]
|
[29] | |||
|
Simethicone
|
API Info |
Irritable bowel syndrome [ICD-11: DD91]
|
[30] | |||
|
Bismuth subsalicylate
|
API Info |
Irritable bowel syndrome [ICD-11: DD91]
|
[31] | |||
|
Ondansetron
|
API Info |
Gastritis [ICD-11: DA42]
|
[32] | |||
|
Dimenhydrinate
|
API Info |
Functional nausea/vomiting [ICD-11: DD90]
|
[33] | |||
|
Lubiprostone
|
API Info |
Irritable bowel syndrome [ICD-11: DD91]
|
[34] | |||
| ICD Disease Classification 14 | Skin disease | Click to Show/Hide | ||||
|
Pseudoephedrine
|
API Info |
Erythema multiforme [ICD-11: EB12]
|
[35] | |||
|
Isotretinoin
|
API Info |
Acne vulgaris [ICD-11: ED80]
|
[36] | |||
|
Demeclocycline
|
API Info |
Acne vulgaris [ICD-11: ED80]
|
[37] | |||
|
Tretinoin
|
API Info |
Acne vulgaris [ICD-11: ED80]
|
[38] | |||
| ICD Disease Classification 15 | Musculoskeletal/connective-tissue disease | Click to Show/Hide | ||||
|
Naproxen
|
API Info |
Rheumatoid arthritis [ICD-11: FA20]
|
[39] | |||
|
Diclofenac
|
API Info |
Knee osteoarthritis [ICD-11: FA01]
|
[40] | |||
|
Cyclosporin A
|
API Info |
Rheumatoid arthritis [ICD-11: FA20]
|
[41] | |||
|
Ibandronic acid
|
API Info |
Osteoporosis [ICD-11: FB83]
|
[42] | |||
|
Tofacitinib
|
API Info |
Rheumatoid arthritis [ICD-11: FA20]
|
[43] | |||
| ICD Disease Classification 16 | Genitourinary system disease | Click to Show/Hide | ||||
|
Ibuprofen
|
API Info |
Dysmenorrhea [ICD-11: GA34]
|
[44] | |||
|
Dutasteride
|
API Info |
Prostatic hyperplasia [ICD-11: GA90]
|
[45] | |||
| ICD Disease Classification 17 | Sexual health-related condition | Click to Show/Hide | ||||
|
Vardenafil
|
API Info |
Erectile dysfunction [ICD-11: HA01]
|
[46] | |||
| ICD Disease Classification 20 | Developmental anomaly | Click to Show/Hide | ||||
|
Calcitriol
|
API Info |
Congenital alopecia [ICD-11: LC30]
|
[47] | |||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.